Current and Future Applications of Computational Fluid Dynamics in Coronary Artery Disease
- PMID: 39076179
- PMCID: PMC11269074
- DOI: 10.31083/j.rcm2311377
Current and Future Applications of Computational Fluid Dynamics in Coronary Artery Disease
Abstract
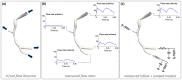
Hemodynamics interacts with the cellular components of human vessels, influencing function and healthy status. Locally acting hemodynamic forces have been associated-by a steadily increasing amount of scientific evidence-with nucleation and evolution of atherosclerotic plaques in several vascular regions, resulting in the formulation of the 'hemodynamic risk hypothesis' of the atherogenesis. At the level of coronary arteries, however, the complexity of both anatomy and physiology made the study of this vascular region particularly difficult for researchers. Developments in computational fluid dynamics (CFD) have recently allowed an accurate modelling of the intracoronary hemodynamics, thus offering physicians a unique tool for the investigation of this crucial human system by means of advanced mathematical simulations. The present review of CFD applications in coronary artery disease was set to concisely offer the medical reader the theoretical foundations of quantitative intravascular hemodynamics-reasoned schematically in the text in its basic (i.e., pressure and velocity) and derived quantities (e.g., fractional flow reserve, wall shear stress and helicity)-along with its current implications in clinical research. Moreover, attention was paid in classifying computational modelling derived from invasive and non-invasive imaging modalities with unbiased remarks on the advantages and limitations of each procedure. Finally, an extensive description-aided by explanatory figures and cross references to recent clinical findings-was presented on the role of near-wall hemodynamics, in terms of shear stress, and of intravascular flow complexity, in terms of helical flow.
Keywords: atherosclerosis; computational hemodynamics; computer model; computer simulation; coronary artery disease; helicity; virtual FFR; wall shear stress.
Copyright: © 2022 The Author(s). Published by IMR Press.
Conflict of interest statement
The author declares no conflict of interest. Fabrizio D’Ascenzo is serving as one of the Editorial Board members of this journal. We declare that Fabrizio D’Ascenzo had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Jerome L. Fleg.
Figures






References
-
- Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. Journal of Interventional Cardiology . 2002;15:439–446. - PubMed
-
- Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A Prospective Natural-History Study of Coronary Atherosclerosis. New England Journal of Medicine . 2011;364:226–235. - PubMed
-
- Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circulation Research . 1990;66:1045–1066. - PubMed
-
- Erlinge D, Maehara A, Ben-Yehuda O, Botker HE, Maeng M, Kjoller-Hansen L, et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet . 2021;397:985–995. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

