Ugi bisamides based on pyrrolyl-β-chlorovinylaldehyde and their unusual transformations
- PMID: 39076293
- PMCID: PMC11285049
- DOI: 10.3762/bjoc.20.156
Ugi bisamides based on pyrrolyl-β-chlorovinylaldehyde and their unusual transformations
Abstract
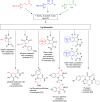
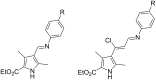
By one-pot four- and three-component Ugi reactions involving convertible isocyanides and unexplored pyrrole-containing β-chlorovinylaldehyde, a small library of 20 bisamides with unusual behavior in post-Ugi transformations was prepared and characterized. Surprisingly, a well-documented approach to obtain peptide-containing carboxylic acids through acid hydrolysis of the convertible isocyanide moiety in the Ugi bisamides proceeded in an unexpected manner in our case, leading to the formation of derivatives of amides of heterylidenepyruvic acid. An optimized synthetic protocol for this transformation was elaborated and a plausible sequence involving the elimination of the 2-chloroacetamide moiety and the conversion of the β-chlorovinyl fragment into a vinyl one is provided.
Keywords: Ugi reaction; convertible isocyanides; multicomponent reaction; post-Ugi transformation; pyrrole derivative.
Copyright © 2024, Tsygankov et al.
Figures








References
-
- Chebanov V A, Desenko S M. Chem Heterocycl Compd. 2012;48:566–583. doi: 10.1007/s10593-012-1030-2. - DOI
-
- Chebanov V A, Desenko S M, Lipson V V, Gorobets N Y. Multicomponent-Switched Reactions in Synthesis of Heterocycles. In: Van der Eycken E V, Sharma U K, editors. Multicomponent Reactions towards Heterocycles: Concepts and Applications. Weinheim, Germany: Wiley-VCH; 2022. pp. 287–338. - DOI
LinkOut - more resources
Full Text Sources
