Intra-articular sprouting of nociceptors accompanies progressive osteoarthritis: comparative evidence in four murine models
- PMID: 39076825
- PMCID: PMC11284167
- DOI: 10.3389/fnana.2024.1429124
Intra-articular sprouting of nociceptors accompanies progressive osteoarthritis: comparative evidence in four murine models
Abstract
Objective: Knee joints are densely innervated by nociceptors. In human knees and rodent models, sprouting of nociceptors has been reported in late-stage osteoarthritis (OA). Here, we sought to describe progressive nociceptor remodeling in early and late-stage OA, using four distinct experimental mouse models.
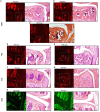
Methods: Sham surgery, destabilization of the medial meniscus (DMM), partial meniscectomy (PMX), or non-invasive anterior cruciate ligament rupture (ACLR) was performed in the right knee of 10-12-week old male C57BL/6 NaV1.8-tdTomato mice. Mice were euthanized (1) 4, 8 or 16 weeks after DMM or sham surgery; (2) 4 or 12 weeks after PMX or sham; (3) 1 or 4 weeks after ACLR injury or sham. Additionally, a cohort of naïve male wildtype mice was evaluated at age 6 and 24 months. Mid-joint cryosections were assessed qualitatively and quantitatively for NaV1.8+ or PGP9.5+ innervation. Cartilage damage, synovitis, and osteophytes were assessed.
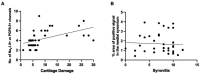
Results: Progressive OA developed in the medial compartment after DMM, PMX, and ACLR. Synovitis and associated neo-innervation of the synovium by nociceptors peaked in early-stage OA. In the subchondral bone, channels containing sprouting nociceptors appeared early, and progressed with worsening joint damage. Two-year old mice developed primary OA in the medial and the lateral compartment, accompanied by nociceptor sprouting in the synovium and the subchondral bone. All four models showed increased nerve signal in osteophytes.
Conclusion: These findings suggest that anatomical neuroplasticity of nociceptors is intrinsic to OA pathology. The detailed description of innervation of the OA joint and its relationship to joint damage might help in understanding OA pain.
Keywords: mouse models; neuroplasticity; nociceptive innervation; osteoarthritis; sprouting.
Copyright © 2024 Obeidat, Ishihara, Li, Adamczyk, Lammlin, Junginger, Maerz, Miller, Miller and Malfait.
Conflict of interest statement
A-MM received consulting fees from Asahi Kasei Pharma Corporation, Orion, and 23andMe. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aso K., Walsh D. A., Wada H., Izumi M., Tomitori H., Fujii K., et al. (2022). Time course and localization of nerve growth factor expression and sensory nerve growth during progression of knee osteoarthritis in rats. Osteoarthr. Cartil. 30, 1344–1355. doi: 10.1016/j.joca.2022.07.003, PMID: - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

