Implications of disease-modifying therapies for multiple sclerosis on immune cells and response to COVID-19 vaccination
- PMID: 39076966
- PMCID: PMC11284103
- DOI: 10.3389/fimmu.2024.1416464
Implications of disease-modifying therapies for multiple sclerosis on immune cells and response to COVID-19 vaccination
Abstract
Introduction: Disease-modifying therapies (DMTs) have been shown to improve disease outcomes in multiple sclerosis (MS) patients. They may also impair the immune response to vaccines, including the SARS-CoV-2 vaccine. However, available data on both the intrinsic immune effects of DMTs and their influence on cellular response to the SARS-CoV-2 vaccine are still incomplete.
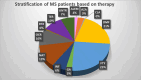
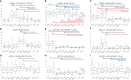
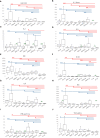
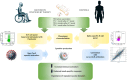
Methods: Here, we evaluated the immune cell effects of 3 DMTs on the response to mRNA SARS-CoV-2 vaccination by comparing MS patients treated with one specific therapy (fingolimod, dimethyl fumarate, or natalizumab) with both healthy controls and untreated patients. We profiled 23 B-cell traits, 57 T-cell traits, and 10 cytokines, both at basal level and after stimulation with a pool of SARS-CoV-2 spike peptides, in 79 MS patients, treated with DMTs or untreated, and 32 healthy controls. Measurements were made before vaccination and at three time points after immunization.
Results and discussion: MS patients treated with fingolimod showed the strongest immune cell dysregulation characterized by a reduction in all measured lymphocyte cell classes; the patients also had increased immune cell activation at baseline, accompanied by reduced specific immune cell response to the SARS-CoV-2 vaccine. Also, anti-spike specific B cells progressively increased over the three time points after vaccination, even when antibodies measured from the same samples instead showed a decline. Our findings demonstrate that repeated booster vaccinations in MS patients are crucial to overcoming the immune cell impairment caused by DMTs and achieving an immune response to the SARS-CoV-2 vaccine comparable to that of healthy controls.
Keywords: SARS-CoV-2; disease-modifying therapy; immune response; immune-phenotyping; multiple sclerosis.
Copyright © 2024 Orrù, Serra, Marongiu, Lai, Lodde, Zoledziewska, Steri, Loizedda, Lobina, Piras, Virdis, Delogu, Marini, Mingoia, Floris, Masala, Castelli, Mostallino, Frau, Lorefice, Farina, Fronza, Carmagnini, Carta, Pilotto, Chessa, Devoto, Castiglia, Solla, Zarbo, Idda, Pitzalis, Cocco, Fiorillo and Cucca.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Krajnc N, Hegen H, Traxler G, Leutmezer F, Di Pauli F, Kornek B, et al. . Humoral immune response to SARS-CoV-2 third vaccination in patients with multiple sclerosis and healthy controls: A prospective multicenter study. Mult Scler Relat Disord. (2022) 65:104009. doi: 10.1016/j.msard.2022.104009 - DOI - PMC - PubMed
-
- König M, Torgauten HM, Tran TT, Holmøy T, Vaage JT, Lund-Johansen F, et al. . Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol. (2022) 79:307–9. doi: 10.1001/jamaneurol.2021.5109 - DOI - PMC - PubMed
-
- Sainz de la Maza S, Walo-Delgado PE, Rodríguez-Domínguez M, Monreal E, Rodero-Romero A, Chico-García JL, et al. . Short- and long-term humoral and cellular immune responses to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with disease-modifying therapies. Vaccines (Basel). (2023) 11:786. doi: 10.3390/vaccines11040786 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

