Neoadjuvant immunochemotherapy improves clinical outcomes of patients with esophageal cancer by mediating anti-tumor immunity of CD8+ T (Tc1) and CD16+ NK cells
- PMID: 39076970
- PMCID: PMC11284045
- DOI: 10.3389/fimmu.2024.1412693
Neoadjuvant immunochemotherapy improves clinical outcomes of patients with esophageal cancer by mediating anti-tumor immunity of CD8+ T (Tc1) and CD16+ NK cells
Abstract
Background: Esophageal cancer (ESCA) is one of the most common tumors in the world, and treatment using neoadjuvant therapy (NT) based on radiotherapy and/or chemotherapy has still unsatisfactory results. Neoadjuvant immunochemotherapy (NICT) has also become an effective treatment strategy nowadays. However, its impact on the tumor microenvironment (TME) and regulatory mechanisms on T cells and NK cells needs to be further elucidated.
Methods: A total of 279 cases of ESCA who underwent surgery alone [non-neoadjuvant therapy (NONE)], neoadjuvant chemotherapy (NCT), and NICT were collected, and their therapeutic effect and survival period were compared. Further, RNA sequencing combined with biological information was used to analyze the expression of immune-related genes. Immunohistochemistry, immunofluorescence, and quantitative real-time PCR (qRT-PCR) were used to verify the activation and infiltration status of CD8+ T and CD16+ NK cells, as well as the function and regulatory pathway of killing tumor cells.
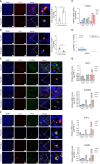
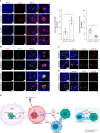
Results: Patients with ESCA in the NICT group showed better clinical response, median survival, and 2-year survival rates (p < 0.05) compared with the NCT group. Our RNA sequencing data revealed that NICT could promote the expression of immune-related genes. The infiltration and activation of immune cells centered with CD8+ T cells were significantly enhanced. CD8+ T cells activated by PD-1 inhibitors secreted more IFN-γ and cytotoxic effector factor cells through the transcription factor of EOMES and TBX21. At the same time, activated CD8+ T cells mediated the CD16+ NK cell activation and secreted more IFN-γ to kill ESCA cells. In addition, the immunofluorescence co-staining results showed that more CD276+ tumor cells and CD16+ NK cells were existed in pre-NCT and pre-NICT group. However, CD276+ tumor cells were reduced significantly in the post-NICT group, while they still appeared in the post-NCT group, which means that CD16+ NK cells can recognize and kill CD276+ tumor cells after immune checkpoint blocker (ICB) treatment.
Conclusion: NICT can improve the therapeutic effect and survival period of resectable ESCA patients. NICT could promote the expression of immune-related genes and activate CD8+ T and CD16+ NK cells to secrete more IFN-γ to kill ESCA cells. It provides a theoretical basis and clinical evidence for its potential as an NT strategy in ESCA.
Keywords: CD16+ NK cells; CD276; CD8+ T cells; esophageal cancer; neoadjuvant immunochemotherapy; tumor microenvironment.
Copyright © 2024 He, Yang, Lin, Zhang, Cheng, Cao, Yang, Zhang, Shi, Jin, Sun, Sun, Zang, Li, Ma and Nie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from globocan 2020. Gastroenterology. (2022) 163:649–58 e2. doi: 10.1053/j.gastro.2022.05.054 - DOI - PubMed
-
- Matsuda S, Kitagawa Y, Takemura R, Okui J, Okamura A, Kawakubo H, et al. Real-world evaluation of the efficacy of neoadjuvant dcf over cf in esophageal squamous cell carcinoma: propensity score-matched analysis from 85 authorized institutes for esophageal cancer in Japan. Ann Surg. (2023) 278:e35–42. doi: 10.1097/SLA.0000000000005533 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

