Unraveling the influence of TTF-1 expression on immunotherapy outcomes in PD-L1-high non-squamous NSCLC: a retrospective multicenter study
- PMID: 39076994
- PMCID: PMC11284020
- DOI: 10.3389/fimmu.2024.1399889
Unraveling the influence of TTF-1 expression on immunotherapy outcomes in PD-L1-high non-squamous NSCLC: a retrospective multicenter study
Abstract
Introduction: Several studies explored the association between thyroid transcription factor-1 (TTF-1) and the therapeutic efficacy of immunotherapy. However, the effect of TTF-1 on the therapeutic efficacy of programmed death-1 (PD-1) inhibitor/chemoimmunotherapy in patients with non-squamous non-small cell lung cancer (non-Sq NSCLC) with a programmed death-ligand 1 (PD-L1) tumor proportion score of 50% or more who are highly susceptible to immunotherapy remains unresolved. Therefore, we evaluated whether TTF-1 has a clinical impact on this population.
Methods: Patients with non-Sq NSCLC and high PD-L1 expression who received PD-1 inhibitor monotherapy or chemoimmunotherapy between May 2017 and December 2020 were retrospectively enrolled. Treatment efficacy was compared after adjusting for baseline differences using propensity score matching.
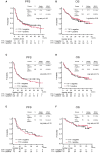
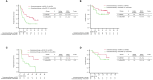
Results: Among the 446 patients with NSCLC with high PD-L1 expression, 266 patients with non-Sq NSCLC were analyzed. No significant differences in therapeutic efficacy were observed between the TTF-1-positive and -negative groups in the overall and propensity score-matched populations. Of chemoimmunotherapy, pemetrexed containing regimen significantly prolonged progression-free survival compared to chemoimmunotherapy without pemetrexed, regardless of TTF-1 expression (TTF1 positive; HR: 0.46 (95% Confidence interval: 0.26-0.81), p<0.01, TTF-1 negative; HR: 0.29 (95% Confidence interval: 0.09-0.93), p=0.02).
Discussion: TTF-1 expression did not affect the efficacy of PD-1 inhibitor monotherapy or chemoimmunotherapy in patients with non-Sq NSCLC with high PD-L1 expression. In this population, pemetrexed-containing chemoimmunotherapy demonstrated superior anti-tumor efficacy, irrespective of TTF-1 expression.
Keywords: PD-L1; TTF-1; antitumor efficacy; immunotherapy; non-squamous non-small cell lung cancer.
Copyright © 2024 Nishioka, Kawachi, Yamada, Tamiya, Negi, Goto, Nakao, Shiotsu, Tanimura, Takeda, Okada, Harada, Date, Chihara, Hasegawa, Tamiya, Masui, Sai, Ishida, Katayama, Morimoto, Iwasaku, Tokuda, Kijima and Takayama.
Conflict of interest statement
NN received personal fees from Chugai Pharmaceutical Co. Ltd., AstraZeneca KK., Eli Lilly Japan KK, and MSD KK outside the purview of the submitted work. HK received personal fees from Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Taiho Pharmaceutical Co. Ltd., Eli Lilly Japan KK, and MSD KK outside the purview of the submitted work. TY received research grants from Ono Pharmaceutical, Janssen, AstraZeneca, and Takeda Pharmaceutical and has received speaking honoraria from Eli Lilly and Chugai Pharmaceutical outside the purview of the submitted work. MT received research grants from Boehringer Ingelheim, Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Daiichi-Sankyo, Eisai, Chugai Pharmaceutical and Janssen, and personal fees from Chugai Pharmaceutical, Boehringer Ingelheim, AstraZeneca, Taiho Pharmaceutical, Eli Lilly, Novartis, Pfizer, Asahi Kasei Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Bayer, Amgen, Kyowa-Kirin, and Nippon Kayaku, outside the purview of the submitted work. AO has received personal fees from Chugai-Roshe, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Japan, Nippon Kayaku, and Bristol Myers Squibb outside the purview of the submitted work. TK received personal fees from Chugai Pharmaceutical Co., Ltd. and MSD KK outside the purview of the submitted work. KCT received research grants from Chugai Pharmaceutical and Ono Pharmaceutical and personal fees from AstraZeneca, Chugai Pharmaceutical, MSD-Merck, Eli Lilly, Boehringer-Ingelheim, and Daiichi-Sankyo outside the purview of the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Elkrief A, Alessi JMV, Ricciuti B, Brown S, Rizvi H, Preeshagul IR, et al. Efficacy of PD-(L)1 blockade monotherapy compared with PD-(L)1 blockade plus chemotherapy in first-line PD-L1-positive advanced lung adenocarcinomas: a cohort study. J Immunother Cancer. (2023);11:e006994. doi: 10.1136/jitc-2023-006994 - DOI - PMC - PubMed
-
- He M, Zheng T, Zhang X, Peng Y, Jiang X, Huang Y, et al. First-line treatment options for advanced non-small cell lung cancer patients with PD-L1 ≥ 50%: a systematic review and network meta-analysis. Cancer Immunol immunotherapy. (2022) 71:1345–55. doi: 10.1007/s00262-021-03089-x - DOI - PMC - PubMed
-
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. (2011) 6:244–85. doi: 10.1097/JTO.0b013e318206a221 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

