New Diagnostic Approach to Arrhythmogenic Cardiomyopathy: The Padua Criteria
- PMID: 39077127
- PMCID: PMC11267345
- DOI: 10.31083/j.rcm2310335
New Diagnostic Approach to Arrhythmogenic Cardiomyopathy: The Padua Criteria
Abstract
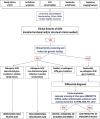
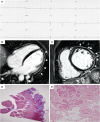
Arrhythmogenic cardiomyopathy (ACM) is a rare heart muscle disease characterized by a progressive fibro-fatty myocardial replacement, ventricular arrhythmias, and increased risk of sudden cardiac death. The first diagnostic criteria were proposed by an International Task Force of experts in 1994 and revised in 2010. At that time, ACM was mainly considered a right ventricle disease, with left ventricle involvement only in the late stages. Since 2010, several pathological and clinical studies using cardiac magnetic resonance (CMR) imaging have allowed to understand the phenotypic expression of the disease and to reach the current idea that ACM may affect both ventricles. Indeed, left ventricular involvement may parallel or exceed right ventricular involvement. The main limitations of the 2010 criteria included the poor sensitivity for left ventricular involvement and the lack of inclusion of tissue characterization by CMR. The 2020 International criteria (the Padua criteria) were developed to overcome these shortcomings. The most important innovations are the introduction of a set of criteria for identifying left ventricular variants and the use of CMR for tissue characterization. Moreover, criteria for right ventricular involvement were also updated taking into account new evidence. According to the number of criteria for right and/or left ventricular involvement, the 2020 Padua criteria allows diagnosing three ACM phenotypic variants: right-dominant, biventricular and left-dominant. This review discusses the evolving approach to diagnosis of ACM, from the 1994 International Criteria to the 2020 Padua criteria.
Keywords: arrhythmogenic cardiomyopathy; cardiac magnetic resonance; cardiomyopathy; diagnosis; ventricular arrhythmias.
Copyright: © 2022 The Author(s). Published by IMR Press.
Conflict of interest statement
The authors declare no conflict of interest. Alessandro Zorzi is serving as one of the Editorial Board members of this journal. We declare that Alessandro Zorzi had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Stefan Peters.
Figures



References
-
- Corrado D, Link MS, Calkins H. Arrhythmogenic Right Ventricular Cardiomyopathy. New England Journal of Medicine . 2017;376:61–72. - PubMed
-
- Maron BJ. Right Ventricular Cardiomyopathy: another Cause of Sudden Death in the Young. New England Journal of Medicine . 1988;318:178–180. - PubMed
-
- Lancisi GM, Caput V, Musca . De motu cordis et aneurysmatibus. Opus posthumu, in duas partes divisum . Naples, Italy: 1736.
-
- Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation . 1982;65:384–398. - PubMed
-
- Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right Ventricular Cardiomyopathy and Sudden Death in Young People. New England Journal of Medicine . 1988;318:129–133. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

