The CorInnova Implantable Cardiac Assist System for Direct Cardiac Compression
- PMID: 39077181
- PMCID: PMC11273667
- DOI: 10.31083/j.rcm2306211
The CorInnova Implantable Cardiac Assist System for Direct Cardiac Compression
Abstract
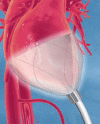
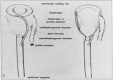
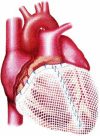
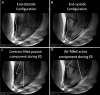
The CorInnova cardiac compression device (CorInnova, Inc., Houston, TX, USA) is designed to provide direct biventricular support, increase cardiac output, and improve ventricular unloading in patients with heart failure. Placed within the pericardium and surrounding both ventricles, the device has two concentric sets of thin-film polyurethane chambers: (1) inner (epicardial) saline-filled chambers that conform intimately to the epicardial surface, eradicating any gaps in the interface between the device and the heart; and (2) outer air-filled chambers cycled to provide epicardial compression during systole and negative epicardial pressure during diastole, consistent with physiological cardiac contraction and relaxation. A superelastic, collapsible Nitinol frame gives the device structure, enables minimally invasive self-deployment, and enhances diastolic filling. Preclinical testing has been extremely promising, with improvements in cardiac output and other cardiac parameters in animal heart failure models. This potentially transformative technology is moving rapidly toward first-in-human use. The CorInnova device may provide an effective device-based solution for patients with heart failure who currently have few or limited mechanical cardiac support options, including patients with biventricular cardiac failure, those with right heart failure, those who are older, and those who are of smaller size. It can be removed easily and requires minimal maintenance. An important, unique feature of this technology is that it provides mechanical cardiac assistance without blood contact or need for anticoagulation. The CorInnova device may be particularly important for those patients who have contraindications to anticoagulation due to allergy, neurological bleeds, or preexisting hemorrhage. No other mechanical circulatory support device addresses these underserved heart-failure populations.
Keywords: assisted circulation; cardiac output; cardiopulmonary resuscitation; heart-assist devices; minimally invasive surgical procedures; stroke volume.
Copyright: © 2022 The Author(s). Published by IMR Press.
Conflict of interest statement
George Letsou is an uncompensated advisor for CorInnova, Inc. and has unrelated financial support from Maquet, Inc. and Terumo Medical Corporation. Christina Bolch, Erica Hord, William Altman, Boris Leschinsky, and John Criscione are employees of CorInnova Inc. and have stock or other remuneration. The authors declare no other conflicts of interest.
Figures


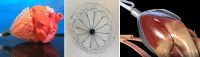

References
-
- Anter E, Jessup M, Callans DJ. Atrial Fibrillation and Heart Failure. Circulation . 2009;119:2516–2525. - PubMed
-
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation . 2018;137:e67–e492. - PubMed
-
- Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-Term Use of a Left Ventricular Assist Device for End-Stage Heart Failure. New England Journal of Medicine . 2001;345:1435–1443. - PubMed
-
- Su A, Al’Aref SJ, Beecy AN, Min JK, Karas MG. Clinical and socioeconomic predictors of heart failure readmissions: a review of contemporary literature. Mayo Clinic Proceedings . 2019;94:1304–1320. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

