Unforeseen and Immediate: A Case of a Post-Watchman Device-Related Thrombus
- PMID: 39077225
- PMCID: PMC11284824
- DOI: 10.7759/cureus.63442
Unforeseen and Immediate: A Case of a Post-Watchman Device-Related Thrombus
Abstract
Atrial fibrillation (AFib) is recognized as a risk factor linked to arterial thromboembolism stemming from blood clot formation in the left atrium, associated with increased morbidity and mortality. Most of these thrombi originate in the left atrial appendage (LAA). Oral anticoagulation (OAC) therapy can help mitigate this risk. LAA occlusion (LAAO) has emerged as an option for patients who cannot safely tolerate long-term OAC. Watchman is one of the commonly used devices with a favorable safety profile demonstrated in numerous studies. One of the most concerning complications of LAAO is device-related thrombus (DRT), which may form on the atrial side of the device and potentially lead to embolization. We present a rare case of immediate DRT formation following the deployment of a Watchman device in a 78-year-old male with persistent AFib. Despite appropriate periprocedural management, a thrombus was observed immediately post implantation. This case emphasizes the need for vigilant surveillance, prompt diagnosis, and therapeutic intervention to manage such complications. The patient was successfully managed with a heparin drip, leading to thrombus resolution. This report underscores the complexities of managing DRT and the importance of ongoing research to optimize outcomes for patients undergoing LAAO.
Keywords: anticoagulation; atrial fibrillation; device-related thrombus; left atrial appendage occlusion; watchman.
Copyright © 2024, Sethi et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
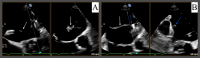
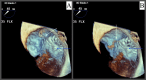
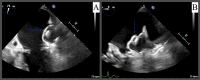
References
-
- Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Dukkipati SR, Kar S, Holmes DR, et al. Circulation. 2018;138:874–885. - PubMed
-
- Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: a meta-analysis. Alkhouli M, Busu T, Shah K, Osman M, Alqahtani F, Raybuck B. JACC Clin Electrophysiol. 2018;4:1629–1637. - PubMed
-
- Incidence, predictors and outcomes of device-related thrombus after left atrial appendage closure with the WATCHMAN device-Insights from the EWOLUTION real world registry. Sedaghat A, Nickenig G, Schrickel JW, et al. Catheter Cardiovasc Interv. 2021;97:0–24. - PubMed
-
- Device-related thrombus after left atrial appendage occlusion: clinical impact, predictors, classification, and management. Alkhouli M, Alarouri H, Kramer A, et al. JACC Cardiovasc Interv. 2023;16:2695–2707. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
