Cardiac Sarcoidosis: A Comprehensive Clinical Review
- PMID: 39077350
- PMCID: PMC11263157
- DOI: 10.31083/j.rcm2502037
Cardiac Sarcoidosis: A Comprehensive Clinical Review
Abstract
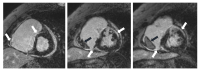
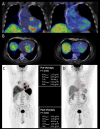
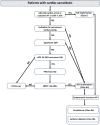
Sarcoidosis is an inflammatory multisystemic disease of unknown etiology characterized by the formation of non-caseating granulomas. Sarcoidosis can affect any organ, predominantly the lungs, lymphatic system, skin and eyes. While 90% of patients with sarcoidosis have lung involvement, an estimated 5% of patients with sarcoidosis have clinically manifest cardiac sarcoidosis (CS), whereas approximately 25% have asymptomatic, clinically silent cardiac involvement verified by autopsy or imaging studies. CS can present with conduction disturbances, ventricular arrhythmias, heart failure or sudden cardiac death. Approximately 30% of 60-year-old patients presenting with unexplained high degree atrioventricular (AV) block or ventricular tachycardia are diagnosed with CS, therefore CS should be strongly considered in such patients. CS is the second leading cause of death among patients affected by sarcoidosis after pulmonary sarcoidosis, therefore its early recognition is important, because early treatment may prevent death from cardiovascular involvement. The establishment of isolated CS diagnosis sometimes can be quite difficult, when extracardiac disease cannot be verified. The other reason for the difficulty to diagnose CS is that CS is a chameleon of cardiology and it can mimic (completely or almost completely) different cardiac diseases, such as arrhythmogenic cardiomyopathy, giant cell myocarditis, dilated, restrictive and hypertrophic cardiomyopathies. In this review article we will discuss the current diagnosis and management of CS and delineate the potential difficulties and pitfalls of establishing the diagnosis in atypical cases of isolated CS.
Keywords: cardiac sarcoidosis; granulomatous disease; sarcoidosis.
Copyright: © 2024 The Author(s). Published by IMR Press.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Alba AC, Gupta S, Kugathasan L, Ha A, Ochoa A, Balter M, et al. Cardiac Sarcoidosis: A Clinical Overview. Current Problems in Cardiology . 2021;46:100936. - PubMed
-
- Iwai K, Tachibana T, Takemura T, Matsui Y, Kitaichi M, Kawabata Y. Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathologica Japonica . 1993;43:372–376. - PubMed
-
- Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Archives of Pathology & Laboratory Medicine . 1995;119:167–172. - PubMed
-
- Drent M, Crouser ED, Grunewald J. Challenges of Sarcoidosis and Its Management. The New England Journal of Medicine . 2021;385:1018–1032. - PubMed
-
- Birnie DH, Kandolin R, Nery PB, Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. European Heart Journal . 2017;38:2663–2670. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

