Many human pharmaceuticals are weak inhibitors of the cytochrome P450 system in rainbow trout (Oncorhynchus mykiss) liver S9 fractions
- PMID: 39077557
- PMCID: PMC11284600
- DOI: 10.3389/ftox.2024.1406942
Many human pharmaceuticals are weak inhibitors of the cytochrome P450 system in rainbow trout (Oncorhynchus mykiss) liver S9 fractions
Abstract
Introduction: Pharmaceutical residues are widely detected in aquatic environment and can be taken up by nontarget species such as fish. The cytochromes P450 (CYP) represent an important detoxification mechanism in fish, like in humans. In the present study, we assessed the correlation of the substrate selectivities of rainbow trout CYP1A and CYP3A homologues with those of human, through determination of the half-maximal inhibitory concentrations (IC50) of a total sixteen human pharmaceuticals toward CYP1A-like ethoxyresorufin O-deethylase (EROD) and CYP3A-like 7-benzyloxy-4-trifluoromethylcoumarin O-debenzylase (BFCOD) in rainbow trout (Oncorhynchus mykiss) liver S9 fractions (RT-S9).
Methods: The inhibitory impacts (IC50) of atomoxetine, atorvastatin, azelastine, bimatoprost, clomethiazole, clozapine, desloratadine, disulfiram, esomeprazole, felbinac, flecainide, orphenadrine, prazosin, quetiapine, sulpiride, and zolmitriptan toward the EROD and BFCOD activities in RT-S9 were determined using the IC50 shift assay, capable of identifying time-dependent inhibitors (TDI). Additionally, the nonspecific binding of the test pharmaceuticals to RT-S9 was assessed using equilibrium dialysis.
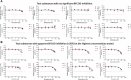
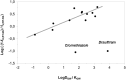
Results: Most test pharmaceuticals were moderate to weak inhibitors of both EROD and BFCOD activity in RT-S9, even if most are noninhibitors of human CYP1A or CYP3A. Only bimatoprost, clomethiazole, felbinac, sulpiride, and zolmitriptan did not inhibit either activity in RT-S9. EROD inhibition was generally stronger than that of BFCOD and some substances (atomoxetine, flecainide, and prazosin) inhibited selectively only EROD activity. The strongest EROD inhibition was detected with azelastine and esomeprazole (unbound IC50 of 3.8 ± 0.5 µM and 3.0 ± 0.8 µM, respectively). None of the test substances were TDIs of BFCOD, but esomeprazole was a TDI of EROD. Apart from clomethiazole and disulfiram, the nonspecific binding of the test pharmaceuticals to the RT-S9 was extensive (unbound fractions <0.5) and correlated well (R 2 = 0.7135) with their water-octanol distribution coefficients.
Discussion: The results indicate that the P450 interactions in RT-S9 cannot be explicitly predicted based on human data, but the in vitro data reported herein can shed light on the substrate selectivity of rainbow trout CYP1A1 and CYP3A27 in comparison to their human homologues. The IC50 concentrations are however many orders of magnitude higher than average environmental concentrations of pharmaceuticals. The time-dependent EROD inhibition by esomeprazole could warrant further research to evaluate its possible interlinkages with hepatotoxic impacts on fish.
Keywords: bioaccumulation; cytochrome P450; ecotoxicology; enzyme inhibition; pharmaceuticals; rainbow trout.
Copyright © 2024 Pihlaja, Oksanen, Vinkvist and Sikanen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor LL declared a past co-authorship with the author TS.
Figures

References
LinkOut - more resources
Full Text Sources

