Cardiovascular Magnetic Resonance for the Evaluation of Arrhythmogenic Substrates in Patients with Systemic Autoimmunity: An Update
- PMID: 39077573
- PMCID: PMC11273118
- DOI: 10.31083/j.rcm2410290
Cardiovascular Magnetic Resonance for the Evaluation of Arrhythmogenic Substrates in Patients with Systemic Autoimmunity: An Update
Abstract
Patients with systemic autoimmunity due to autoimmune rheumatic diseases (ARDs) or sarcoidosis frequently present with systemic manifestations including cardiac involvement. Cardiac rhythm disturbances and specifically ventricular arrhythmias (VAs) may affect the prognosis of these patients. Cardiovascular magnetic resonance imaging (CMR) is a non-invasive imaging modality that can provide valuable diagnostic and prognostic information in patients with ARDs or systemic autoimmunity in general. In this narrative review, we briefly present the underlying pathophysiologic mechanisms contributing to arrhythmogenicity in patients with systemic autoimmunity. Furthermore, we discuss recent advances underlying the role and value of CMR for use in the detection and risk stratification of arrhythmogenic substrates in patients with systemic autoimmunity and VAs.
Keywords: autoimmune disease; cardiovascular disease; fibrosis; inflammation; ischemia; oedema.
Copyright: © 2023 The Author(s). Published by IMR Press.
Conflict of interest statement
The authors declare no conflict of interest. Sophie I. Mavrogeni is serving as Guest Editor and Editorial Board of this journal. We declare that Sophie I. Mavrogeni had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Zhonghua Sun.
Figures

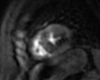


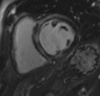

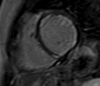

References
-
- Gawałko M, Balsam P, Lodziński P, Grabowski M, Krzowski B, Opolski G, et al. Cardiac Arrhythmias in Autoimmune Diseases. Circulation Journal . 2020;84:685–694. - PubMed
-
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, Koutsogeorgopoulou L, Markousis-Mavrogenis G, Poulos G, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatology International . 2018;38:1615–1621. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

