Overview of Inorganic Electrolytes for All-Solid-State Sodium Batteries
- PMID: 39078042
- PMCID: PMC11325648
- DOI: 10.1021/acs.langmuir.4c01845
Overview of Inorganic Electrolytes for All-Solid-State Sodium Batteries
Abstract
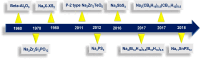
All-solid-state sodium batteries (AS3B) emerged as a strong contender in the global electrochemical energy storage market as a replacement for current lithium-ion batteries (LIB) owing to their high abundance, low cost, high safety, high energy density, and long calendar life. Inorganic electrolytes (IEs) are highly preferred over the conventional liquid and solid polymer electrolytes for sodium-ion batteries (SIBs) due to their high ionic conductivity (∼10-2-10-4 S cm-1), wide potential window (∼5 V), and overall better battery performances. This review discusses the bird's eye view of the recent progress in inorganic electrolytes such as Na-β"-alumina, NASICON, sulfides, antipervoskites, borohydride-type electrolytes, etc. for AS3Bs. Current state-of-the-art inorganic electrolytes in correlation with their ionic conduction mechanism present challenges and interfacial characteristics that have been critically reviewed in this review. The current challenges associated with the present battery configuration are overlooked, and also the chemical and electrochemical stabilities are emphasized. The substantial solution based on ongoing electrolyte development and promising modification strategies are also suggested.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











References
-
- Akimbekov N. S.; Digel I.; Marzhan K.; Tastambek K. T.; Sherelkhan D. K.; Qiao X. Microbial Co-processing and Beneficiation of Low-rank Coals for Clean Fuel Production: A Review. Engineered Science 2023, 25, 942. 10.30919/es942. - DOI
-
- Wang J.; Fu R.; Wen S.; Ning P.; Helal M. H.; Salem M. A.; Xu B. B.; El-Bahy Z. M.; Huang M.; Guo Z.; Huang L.; Wang Q. Progress and current challenges for CO2 capture materials from ambient air. Advanced Composites and Hybrid Materials 2022, 5 (4), 2721–2759. 10.1007/s42114-022-00567-3. - DOI
-
- Zhao J.; Wang B.; Zhan Z.; Hu M.; Cai F.; Świerczek K.; Yang K.; Ren J.; Guo Z.; Wang Z. Boron-doped three-dimensional porous carbon framework/carbon shell encapsulated silicon composites for high-performance lithium-ion battery anodes. J. Colloid Interface Sci. 2024, 664, 790–800. 10.1016/j.jcis.2024.03.053. - DOI - PubMed
-
- Niu H.; Ding M.; Zhang N.; Guo X.; Guan P.; Hu X. Ionic Liquid-Modified Silicon Nanoparticles Composite Gel Polymer Electrolyte for High-Performance Lithium Batteries. ChemElectroChem. 2023, 10 (3), e202201015. 10.1002/celc.202201015. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

