MS-20 enhances the gut microbiota-associated antitumor effects of anti-PD1 antibody
- PMID: 39078050
- PMCID: PMC11290773
- DOI: 10.1080/19490976.2024.2380061
MS-20 enhances the gut microbiota-associated antitumor effects of anti-PD1 antibody
Abstract
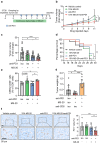
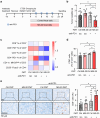
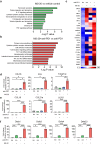
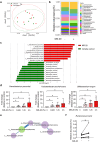
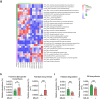
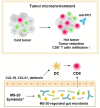
Cancer immunotherapy has been regarded as a promising strategy for cancer therapy by blocking immune checkpoints and evoking immunity to fight cancer, but its efficacy seems to be heterogeneous among patients. Manipulating the gut microbiota is a potential strategy for enhancing the efficacy of immunotherapy. Here, we report that MS-20, also known as "Symbiota®", a postbiotic that comprises abundant microbial metabolites generated from a soybean-based medium fermented with multiple strains of probiotics and yeast, inhibited colon and lung cancer growth in combination with an anti-programmed cell death 1 (PD1) antibody in xenograft mouse models. Mechanistically, MS-20 remodeled the immunological tumor microenvironment by increasing effector CD8+ T cells and downregulating PD1 expression, which were mediated by the gut microbiota. Fecal microbiota transplantation (FMT) from mice receiving MS-20 treatment to recipient mice increased CD8+ T-cell infiltration into the tumor microenvironment and significantly improved antitumor activity when combined with anti-PD1 therapy. Notably, the abundance of Ruminococcus bromii, which increased following MS-20 treatment, was positively associated with a reduced tumor burden and CD8+ T-cell infiltration in vivo. Furthermore, an ex vivo study revealed that MS-20 could alter the composition of the microbiota in cancer patients, resulting in distinct metabolic pathways associated with favorable responses to immunotherapy. Overall, MS-20 could act as a promising adjuvant agent for enhancing the efficacy of immune checkpoint-mediated antitumor therapy.
Keywords: Gut microbiota; cancer immunotherapy; colorectal cancer.
Conflict of interest statement
The authors declare the following potential conflicts of interest: PJL, CMH, AJY, CYH, WCC, WYH, WCK, KML, MLK and WJC are employed by Microbio Co., Ltd.; YCC and CCY are employed by Microbio (Shanghai) Biotech Company; HWC is employed by Oneness Biotech; and HCL is employed by Revivebio Co., Ltd.
Figures

References
-
- Pons-Tostivint E, Latouche A, Vaflard P, Ricci F, Loirat D, Hescot S, Sablin M-P, Rouzier R, Kamal M, Morel C, et al. Comparative analysis of durable responses on immune checkpoint inhibitors versus other systemic therapies: a pooled analysis of phase III trials. JCO Precis Oncol. 2019;3(3):1–21. doi:10.1200/PO.18.00114. - DOI - PubMed
-
- Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Man Lei Y, Jabri B, Alegre M-L, et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi:10.1126/science.aac4255. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
