NaV1.5 autoantibodies in Brugada syndrome: pathogenetic implications
- PMID: 39078224
- PMCID: PMC11491155
- DOI: 10.1093/eurheartj/ehae480
NaV1.5 autoantibodies in Brugada syndrome: pathogenetic implications
Abstract
Background and aims: Patients suffering from Brugada syndrome (BrS) are predisposed to life-threatening cardiac arrhythmias. Diagnosis is challenging due to the elusive electrocardiographic (ECG) signature that often requires unconventional ECG lead placement and drug challenges to be detected. Although NaV1.5 sodium channel dysfunction is a recognized pathophysiological mechanism in BrS, only 25% of patients have detectable SCN5A variants. Given the emerging role of autoimmunity in cardiac ion channel function, this study explores the presence and potential impact of anti-NaV1.5 autoantibodies in BrS patients.
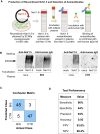
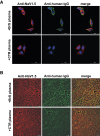
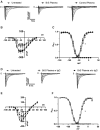
Methods: Using engineered HEK293A cells expressing recombinant NaV1.5 protein, plasma from 50 BrS patients and 50 controls was screened for anti-NaV1.5 autoantibodies via western blot, with specificity confirmed by immunoprecipitation and immunofluorescence. The impact of these autoantibodies on sodium current density and their pathophysiological effects were assessed in cellular models and through plasma injection in wild-type mice.
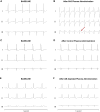
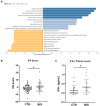
Results: Anti-NaV1.5 autoantibodies were detected in 90% of BrS patients vs. 6% of controls, yielding a diagnostic area under the curve of .92, with 94% specificity and 90% sensitivity. These findings were consistent across varying patient demographics and independent of SCN5A mutation status. Electrophysiological studies demonstrated a significant reduction specifically in sodium current density. Notably, mice injected with BrS plasma showed Brugada-like ECG abnormalities, supporting the pathogenic role of these autoantibodies.
Conclusions: The study demonstrates the presence of anti-NaV1.5 autoantibodies in the majority of BrS patients, suggesting an immunopathogenic component of the syndrome beyond genetic predispositions. These autoantibodies, which could serve as additional diagnostic markers, also prompt reconsideration of the underlying mechanisms of BrS, as evidenced by their role in inducing the ECG signature of the syndrome in wild-type mice. These findings encourage a more comprehensive diagnostic approach and point to new avenues for therapeutic research.
Keywords: Autoantibodies; Biomarker; Brugada syndrome; NaV1.5.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

References
-
- Milman A, Hochstadt A, Andorin A, Gourraud JB, Sacher F, Mabo P, et al. . Time-to-first appropriate shock in patients implanted prophylactically with an implantable cardioverter-defibrillator: data from the Survey on Arrhythmic Events in BRUgada Syndrome (SABRUS). Europace 2019;21:796–802. 10.1093/europace/euy301 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

