Pharmacogenomic Score Effectively Personalizes Treatment of Acute Myeloid Leukemia
- PMID: 39078289
- PMCID: PMC11444877
- DOI: 10.1158/1078-0432.CCR-24-0863
Pharmacogenomic Score Effectively Personalizes Treatment of Acute Myeloid Leukemia
Abstract
Purpose: Cytarabine (also known as ara-C) has been the backbone of acute myeloid leukemia (AML) chemotherapy for more than five decades. Recent pharmacogenomics-based 10-SNP ara-C (ACS10) scores showed low ACS10 (≤0) to be associated with poor outcomes in patients with AML treated with standard chemotherapy. Here, we evaluated the ACS10 score in the context of three different induction I regimens in patients with pediatric AML.
Experimental design: ACS10 score groups (low, ≤0, or high, >0) were evaluated for association with event-free survival (EFS) and overall survival (OS) by three randomized treatment arms in patients treated on the AML02 (NCT00136084) and AML08 (NCT00703820) clinical trials: AML02 low-dose ara-C (LDAC arm, n = 91), AML02 + AML08 high-dose ara-C (HDAC arm, n = 194), and AML08 clofarabine + ara-C (Clo/ara-C arm, n = 105) induction I regimens.
Results: Within the low-ACS10 score (≤0) group, significantly improved EFS and OS were observed among patients treated with Clo/ara-C as compared with LDAC (EFS, HR = 0.45; 95% CI, 0.23-0.88; P = 0.020; OS, HR = 0.44; 95% CI, 0.19-0.99; P = 0.048). In contrast, within the high-ACS10 score group (score >0), augmentation with Clo/ara-C was not favorable as compared with LDAC (Clo/ara-C vs. LDAC, EFS, HR = 1.95; 95% CI, 1.05-3.63; P = 0.035; OS, HR = 2.10; 95% CI, 0.96-4.59; P = 0.063). Personalization models predicted 9% improvement in the outcome in ACS10 score-based tailored induction (Clo/ara-C for low and LDAC for high-ACS10 score groups) as compared with nonpersonalized approaches (P < 0.002).
Conclusions: Our findings suggest that tailoring induction regimens using ACS10 scores can significantly improve outcomes in patients with AML. Given the SNPs are germline, preemptive genotyping can accelerate matching the most effective remission induction regimen.
©2024 American Association for Cancer Research.
Conflict of interest statement
Figures



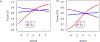

References
-
- Bose P, Vachhani P, Cortes JE: Treatment of Relapsed/Refractory Acute Myeloid Leukemia. Curr Treat Options Oncol 18:17, 2017 - PubMed
-
- Dohner H, Weisdorf DJ, Bloomfield CD: Acute Myeloid Leukemia. N Engl J Med 373:1136–52, 2015 - PubMed
-
- Rubnitz JE, Kaspers GJL: How I treat pediatric acute myeloid leukemia. Blood 138:1009–1018, 2021 - PubMed
-
- Chou TC, Arlin Z, Clarkson BD, et al.: Metabolism of 1-beta-D-arabinofuranosylcytosine in human leukemic cells. Cancer Res 37:3561–70, 1977 - PubMed
-
- Grant S: Ara-C: cellular and molecular pharmacology. Adv Cancer Res 72:197–233, 1998 - PubMed

