Intratumoral Injection of Immunotherapeutics: State of the Art and Future Directions
- PMID: 39078294
- PMCID: PMC11294769
- DOI: 10.1148/radiol.232654
Intratumoral Injection of Immunotherapeutics: State of the Art and Future Directions
Abstract
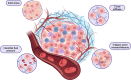
Systemic immunotherapies have led to tremendous progress across the cancer landscape. However, several challenges exist, potentially limiting their efficacy in the treatment of solid tumors. Direct intratumoral injection can increase the therapeutic index of immunotherapies while overcoming many of the barriers associated with systemic administration, including limited bioavailability to tumors and potential systemic safety concerns. However, challenges remain, including the lack of standardized approaches for administration, issues relating to effective drug delivery, logistical hurdles, and safety concerns specific to this mode of administration. This article reviews the biologic rationale for the localized injection of immunotherapeutic agents into tumors. It also addresses the existing limitations and practical considerations for safe and effective implementation and provide recommendations for optimizing logistics and treatment workflows. It also highlights the critical role that radiologists, interventional radiologists, and medical physicists play in intratumoral immunotherapy with respect to target selection, image-guided administration, and response assessment.
© RSNA, 2024.
Conflict of interest statement
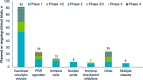
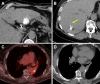
Figures



References
-
- Schmid P , Adams S , Rugo HS , et al . Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer . N Engl J Med 2018. ; 379 ( 22 ): 2108 – 2121 . - PubMed
-
- Forde PM , Chaft JE , Pardoll DM . Neoadjuvant PD-1 blockade in resectable lung cancer . N Engl J Med 2018. ; 379 ( 9 ): e14 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

