Pharmacologic Inhibition of EIF4A Blocks NRF2 Synthesis to Prevent Osteosarcoma Metastasis
- PMID: 39078310
- PMCID: PMC11443218
- DOI: 10.1158/1078-0432.CCR-24-1317
Pharmacologic Inhibition of EIF4A Blocks NRF2 Synthesis to Prevent Osteosarcoma Metastasis
Abstract
Purpose: Effective therapies for metastatic osteosarcoma (OS) remain a critical unmet need. Targeting mRNA translation in metastatic OS offers a promising option, as selective translation drives the synthesis of cytoprotective proteins under harsh microenvironmental conditions to facilitate metastatic competence.
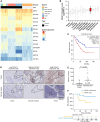
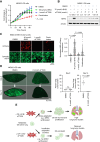
Experimental design: We assessed the expression levels of eukaryotic translation factors in OS, revealing the high expression of the eukaryotic initiation factor 4A1 (EIF4A1). Using a panel of metastatic OS cell lines and patient-derived xenograft (PDX) models, EIF4A1 inhibitors were evaluated for their ability to block proliferation and reduce survival under oxidative stress, mimicking harsh conditions of the lung microenvironment. Inhibitors were also evaluated for their antimetastatic activity using the ex vivo pulmonary metastasis assay and in vivo metastasis models. Proteomics was performed to catalog which cytoprotective proteins or pathways were affected by EIF4A1 inhibition.
Results: CR-1-31B, a rocaglate-based EIF4A1 inhibitor, exhibited nanomolar cytotoxicity against all metastatic OS models tested. CR-1-31B exacerbated oxidative stress and apoptosis when OS cells were co-treated with tert-butylhydroquinone, a chemical oxidative stress inducer. CR-1-31B potently inhibited OS growth in the pulmonary metastasis assay model and in experimental and spontaneous models of OS lung metastasis. Proteomic analysis revealed that tert-butylhydroquinone-mediated upregulation of the NRF2 antioxidant factor was blocked by co-treatment with CR-1-31B. Genetic inactivation of NRF2 phenocopied the antimetastatic activity of CR-1-31B. Finally, the clinical-grade EIF4A1 phase-1-to-2 inhibitor, zotatifin, similarly blocked NRF2 synthesis and the OS metastatic phenotype.
Conclusions: Collectively, our data reveal that pharmacologic targeting of EIF4A1 is highly effective in blocking OS metastasis by blunting the NRF2 antioxidant response.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
M.M. Lizardo reports nonfinancial support from eFFECTOR Therapeutics during the conduct of the study. E. Sweet-Cordero reports grants from NCI during the conduct of the study. No disclosures were reported by the other authors.
Figures




References
-
- Belayneh R, Fourman MS, Bhogal S, Weiss KR. Update on osteosarcoma. Curr Oncol Rep 2021;23:71. - PubMed
-
- Koshkina N, Yang Y, Kleinerman ES. The Fas/FasL signaling pathway: its role in the metastatic process and as a target for treating osteosarcoma lung metastases. In: Kleinerman ES, Gorlick R, editors. Current Advances in the Science of Osteosarcoma: Research Perspectives: Tumor Biology, Organ Microenvironment, Potential New Therapeutic Targets, and Canine Models. Cham: Springer International Publishing; 2020. pp. 177–87.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

