Effects of semaglutide, empagliflozin and their combination on renal diffusion-weighted MRI and total kidney volume in patients with type 2 diabetes: a post hoc analysis from a 32 week randomised trial
- PMID: 39078489
- PMCID: PMC11447057
- DOI: 10.1007/s00125-024-06228-y
Effects of semaglutide, empagliflozin and their combination on renal diffusion-weighted MRI and total kidney volume in patients with type 2 diabetes: a post hoc analysis from a 32 week randomised trial
Abstract
Aims/hypothesis: The apparent diffusion coefficient (ADC) derived from diffusion-weighted MRI (DWI-MRI) has been proposed as a measure of changes in kidney microstructure, including kidney fibrosis. In advanced kidney disease, the kidneys often become atrophic; however, in the initial phase of type 2 diabetes, there is an increase in renal size. Glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors both provide protection against progression of kidney disease in diabetes. However, the mechanisms are incompletely understood. To explore this, we examined the effects of semaglutide, empagliflozin and their combination on renal ADC and total kidney volume (TKV).
Methods: This was a substudy of a randomised clinical trial on the effects of semaglutide and empagliflozin alone or in combination. Eighty patients with type 2 diabetes and high risk of CVD were randomised into four groups (n=20 in each) receiving either tablet placebo, empagliflozin, a combination of semaglutide and tablet placebo (herein referred to as the 'semaglutide' group), or the combination of semaglutide and empagliflozin (referred to as the 'combination-therapy' group). The semaglutide and the combination-therapy group had semaglutide treatment for 16 weeks and then had either tablet placebo or empagliflozin added to the treatment, respectively, for a further 16 weeks; the placebo and empagliflozin groups were treated with the respective monotherapy for 32 weeks. We analysed the effects of treatment on changes in ADC (cortical, medullary and the cortico-medullary difference [ΔADC; medullary ADC subtracted from cortical ADC]), as well as TKV measured by MRI.
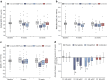
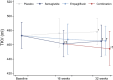
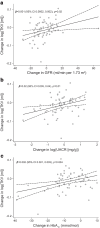
Results: Both semaglutide and empagliflozin decreased cortical ADC significantly compared with placebo (semaglutide: -0.20×10-3 mm2/s [95% CI -0.30, -0.10], p<0.001; empagliflozin: -0.15×10-3 mm2/s [95% CI -0.26, -0.04], p=0.01). No significant change was observed in the combination-therapy group (-0.05×10-3 mm2/s [95%CI -0.15, 0.05]; p=0.29 vs placebo). The changes in cortical ADC were not associated with changes in GFR, albuminuria, TKV or markers of inflammation. Further, there were no changes in medullary ADC in any of the groups compared with placebo. Only treatment with semaglutide changed ΔADC significantly from placebo, showing a decrease of -0.13×10-3 mm2/s (95% CI -0.22, -0.04; p=0.01). Compared with placebo, TKV decreased by -3% (95% CI -5%, -0.3%; p=0.04), -3% (95% CI -5%, -0.4%; p=0.02) and -5% (95% CI -8%, -2%; p<0.001) in the semaglutide, empagliflozin and combination-therapy group, respectively. The changes in TKV were associated with changes in GFR, albuminuria and HbA1c.
Conclusions/interpretation: In a population with type 2 diabetes and high risk of CVD, semaglutide and empagliflozin significantly reduced cortical ADC compared with placebo, indicating microstructural changes in the kidneys. These changes were not associated with changes in GFR, albuminuria or inflammation. Further, we found a decrease in TKV in all active treatment groups, which was possibly mediated by a reduction in hyperfiltration. Our findings suggest that DWI-MRI may serve as a promising tool for investigating the underlying mechanisms of medical interventions in individuals with type 2 diabetes but may reflect effects not related to fibrosis.
Trial registration: European Union Drug Regulating Authorities Clinical Trials Database (EudraCT) 2019-000781-38.
Keywords: Apparent diffusion coefficient; Diffusion-weighted magnetic resonance imaging; Glucagon-like peptide-1 receptor agonist; Magnetic resonance imaging; Sodium–glucose cotransporter 2 inhibitors; Total kidney volume; Type 2 diabetes.
© 2024. The Author(s).
Figures

References
-
- Palmer SC, Tendal B, Mustafa RA et al (2021) Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 372:m4573. 10.1136/bmj.m4573 - PMC - PubMed
-
- Sattar N, Lee MMY, Kristensen SL et al (2021) Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 9(10):653–662. 10.1016/s2213-8587(21)00203-5 - PubMed
-
- Perkovic V, Jardine MJ, Neal B et al (2019) Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380(24):2295–2306. 10.1056/NEJMoa1811744 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

