Medication Optimization Protocol Efficacy for Geriatric Inpatients: A Randomized Clinical Trial
- PMID: 39078632
- PMCID: PMC11289701
- DOI: 10.1001/jamanetworkopen.2024.23544
Medication Optimization Protocol Efficacy for Geriatric Inpatients: A Randomized Clinical Trial
Erratum in
-
Error in Figure 1.JAMA Netw Open. 2024 Nov 4;7(11):e2449465. doi: 10.1001/jamanetworkopen.2024.49465. JAMA Netw Open. 2024. PMID: 39531239 Free PMC article. No abstract available.
Abstract
Importance: There is currently no consensus on clinically effective interventions for polypharmacy among older inpatients.
Objective: To evaluate the effect of multidisciplinary team-based medication optimization on survival, unscheduled hospital visits, and rehospitalization in older inpatients with polypharmacy.
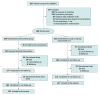
Design, setting, and participants: This open-label randomized clinical trial was conducted at 8 internal medicine inpatient wards within a community hospital in Japan. Participants included medical inpatients 65 years or older who were receiving 5 or more regular medications. Enrollment took place between May 21, 2019, and March 14, 2022. Statistical analysis was performed from September 2023 to May 2024.
Intervention: The participants were randomly assigned to receive either an intervention for medication optimization or usual care including medication reconciliation. The intervention consisted of a medication review using the STOPP (Screening Tool of Older Persons' Prescriptions)/START (Screening Tool to Alert to Right Treatment) criteria, followed by a medication optimization proposal for participants and their attending physicians developed by a multidisciplinary team. On discharge, the medication optimization summary was sent to patients' primary care physicians and community pharmacists.
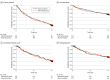
Main outcomes and measures: The primary outcome was a composite of death, unscheduled hospital visits, and rehospitalization within 12 months. Secondary outcomes included the number of prescribed medications, falls, and adverse events.
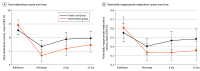
Results: Between May 21, 2019, and March 14, 2022, 442 participants (mean [SD] age, 81.8 [7.1] years; 223 [50.5%] women) were randomly assigned to the intervention (n = 215) and usual care (n = 227). The intervention group had a significantly lower percentage of patients with 1 or more potentially inappropriate medications than the usual care group at discharge (26.2% vs 33.0%; adjusted odds ratio [OR], 0.56 [95% CI, 0.33-0.94]; P = .03), at 6 months (27.7% vs 37.5%; adjusted OR, 0.50 [95% CI, 0.29-0.86]; P = .01), and at 12 months (26.7% vs 37.4%; adjusted OR, 0.45 [95% CI, 0.25-0.80]; P = .007). The primary composite outcome occurred in 106 participants (49.3%) in the intervention group and 117 (51.5%) in the usual care group (stratified hazard ratio, 0.98 [95% CI, 0.75-1.27]). Adverse events were similar between each group (123 [57.2%] in the intervention group and 135 [59.5%] in the usual care group).
Conclusions and relevance: In this randomized clinical trial of older inpatients with polypharmacy, the multidisciplinary deprescribing intervention did not reduce death, unscheduled hospital visits, or rehospitalization within 12 months. The intervention was effective in reducing the number of medications with no significant adverse effects on clinical outcomes, even among older inpatients with polypharmacy.
Trial registration: UMIN Clinical Trials Registry: UMIN000035265.
Conflict of interest statement
Figures
Comment in
-
Building an Evidence Base for Deprescribing in the Setting of Polypharmacy.JAMA Netw Open. 2024 Jul 1;7(7):e2423529. doi: 10.1001/jamanetworkopen.2024.23529. JAMA Netw Open. 2024. PMID: 39078638 No abstract available.
References
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources

