In utero delivery of targeted ionizable lipid nanoparticles facilitates in vivo gene editing of hematopoietic stem cells
- PMID: 39078677
- PMCID: PMC11317576
- DOI: 10.1073/pnas.2400783121
In utero delivery of targeted ionizable lipid nanoparticles facilitates in vivo gene editing of hematopoietic stem cells
Abstract
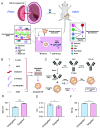
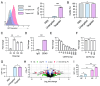
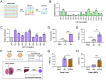
Monogenic blood diseases are among the most common genetic disorders worldwide. These diseases result in significant pediatric and adult morbidity, and some can result in death prior to birth. Novel ex vivo hematopoietic stem cell (HSC) gene editing therapies hold tremendous promise to alter the therapeutic landscape but are not without potential limitations. In vivo gene editing therapies offer a potentially safer and more accessible treatment for these diseases but are hindered by a lack of delivery vectors targeting HSCs, which reside in the difficult-to-access bone marrow niche. Here, we propose that this biological barrier can be overcome by taking advantage of HSC residence in the easily accessible liver during fetal development. To facilitate the delivery of gene editing cargo to fetal HSCs, we developed an ionizable lipid nanoparticle (LNP) platform targeting the CD45 receptor on the surface of HSCs. After validating that targeted LNPs improved messenger ribonucleic acid (mRNA) delivery to hematopoietic lineage cells via a CD45-specific mechanism in vitro, we demonstrated that this platform mediated safe, potent, and long-term gene modulation of HSCs in vivo in multiple mouse models. We further optimized this LNP platform in vitro to encapsulate and deliver CRISPR-based nucleic acid cargos. Finally, we showed that optimized and targeted LNPs enhanced gene editing at a proof-of-concept locus in fetal HSCs after a single in utero intravenous injection. By targeting HSCs in vivo during fetal development, our Systematically optimized Targeted Editing Machinery (STEM) LNPs may provide a translatable strategy to treat monogenic blood diseases before birth.
Keywords: CRISPR; congenital disease; hematopoietic stem cell; lipid nanoparticles; mRNA.
Conflict of interest statement
Competing interests statement:R.P., M.J.M., and W.H.P. are inventors on a U.S. Provisional Patent Application (No. 63/623,674) related to the technology described in the manuscript.
Figures


References
-
- Chui D. H. K., Waye J. S., Hydrops fetalis caused by α-thalassemia: An emerging health care problem. Blood 91, 2213–2222 (1998). - PubMed
MeSH terms
Substances
Grants and funding
- F30HL162465-01A1/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01DK123049/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R01 DK123049/DK/NIDDK NIH HHS/United States
- DP2 TR002776/TR/NCATS NIH HHS/United States
- U19 NS132301/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

