Effectiveness of PARP Inhibitor Maintenance Therapy in Ovarian Cancer by BRCA1/2 and a Scar-Based HRD Signature in Real-World Practice
- PMID: 39078736
- PMCID: PMC11474169
- DOI: 10.1158/1078-0432.CCR-24-1225
Effectiveness of PARP Inhibitor Maintenance Therapy in Ovarian Cancer by BRCA1/2 and a Scar-Based HRD Signature in Real-World Practice
Erratum in
-
Correction: Effectiveness of PARP Inhibitor Maintenance Therapy in Ovarian Cancer by BRCA1/2 and a Scar-Based HRD Signature in Real-World Practice.Clin Cancer Res. 2025 Aug 14;31(16):3599. doi: 10.1158/1078-0432.CCR-25-2196. Clin Cancer Res. 2025. PMID: 40808406 Free PMC article. No abstract available.
Abstract
Purpose: The purpose of the study was to compare the effectiveness of PARP inhibitor maintenance therapy (mPARPi) in real-world practice by biomarker status [BRCA1/2 alterations (BRCAalt) and a homologous recombination deficiency signature (HRDsig)] in advanced ovarian cancer.
Experimental design: Patients with ovarian cancer receiving first-line platinum-based chemotherapy and either mPARPi or no maintenance were included. Patient data were obtained by a US-based de-identified ovarian cancer Clinico-Genomic Database, from ∼280 US cancer clinics (01/2015-03/2023). Real-world progression-free survival (rwPFS) and overall survival (rwOS) were compared by biomarker status using Cox models, weighted by propensity scores.
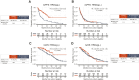
Results: Of 673 patients, 160 received mPARPi [31.2% BRCAalt and 51.9% HRDsig(+)] and 513 no maintenance [15.6% BRCAalt and 34.1% HRDsig(+)]. BRCAalt patients receiving mPARPi versus no maintenance had favorable rwPFS [HR, 0.48; 95% confidence interval (CI), 0.26-0.87; P = 0.0154], as did BRCA wild-type (WT; HR, 0.76; 95% CI, 0.57-1.01; P = 0.0595). Favorable rwOS was not observed with mPARPi for BRCAalt or BRCA-WT. HRDsig(+) patients receiving mPARPi versus no maintenance had favorable rwPFS (HR, 0.36; 95% CI, 0.24-0.55; P < 0.001) and numerically favorable rwOS (HR, 0.46; 95% CI, 0.21-1.02; P = 0.0561). No differences were observed for HRDsig(-). mPARPi treatment interaction was observed for HRDsig(+) versus HRDsig(-) (rwPFS P < 0.001/rwOS P = 0.016) but not for BRCAalt versus BRCA-WT. Patients with BRCA-WT/HRDsig(+) receiving mPARPi had favorable rwPFS (HR, 0.40; 95% CI, 0.22-0.72; P = 0.003), whereas no difference was observed for BRCA-WT/HRDsig(-).
Conclusions: HRDsig predicted benefit of mPARPi better than BRCAalt. Patients with HRDsig(+) status experienced favorable outcomes, even if they had BRCA-WT status. In contrast, patients with HRDsig(-) status did not show significant benefit from mPARPi treatment. HRDsig might predict benefit from mPARPi regardless of BRCAalt status.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
D.L. Richardson reports personal fees from AstraZeneca, Mersana, Daiichi Sankyo, ProfoundBio, Eisai, and ImmuoGen and grants and personal fees from GlaxoSmithKline outside the submitted work. J.C.F. Quintanilha reports other support from Foundation Medicine, Inc and Roche during the conduct of the study. N. Danziger reports other support from Foundation Medicine, Inc and Roche during the conduct of the study. G. Li reports personal fees from Foundation Medicine, Inc and other support from Roche outside the submitted work. E.S. Sokol reports other support from Foundation Medicine and other support from Roche outside the submitted work. A.B. Schrock reports personal fees from Foundation Medicine and other support from Roche during the conduct of the study. E.M. Ebot reports personal fees from Foundation Medicine outside the submitted work and is a stockholder of Roche Holdings AG. N. Bhardwaj reports other support from Foundation Medicine outside the submitted work. A. Afghani reports other support from Roche during the conduct of the study, as well as other support from Roche outside the submitted work. C. Washington reports personal fees from AstraZeneca and grants from Robert A Winn Foundation STAAR outside the submitted work. J.A. Elvin reports full-time employment with Foundation Medicine, Inc. Foundation Medicine is an independent affiliate of the Roche Group, which provides equity interests as part of its employee benefit package. R.P. Graf reports other support from Foundation Medicine, Inc during the conduct of the study, as well as other support from Roche outside the submitted work. K.N. Moore reports personal fees from AstraZeneca, Aadi, Blueprint Pharma, Caris, Duality, BioNTech, Eisai, GSK, ImmunoGen, Janssen, Eli Lilly and Company, Merck, Novartis, Regeneron, Verastem, and Zentalis outside the submitted work. No disclosures were reported by the other authors.
Figures



References
-
- Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018;379:2495–505. - PubMed
-
- Gonzalez-Martin A, Pothuri B, Vergote I, Graybill W, Lorusso D, McCormick CC, et al. Progression-free survival and safety at 3.5years of follow-up: results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer. Eur J Cancer 2023;189:112908. - PubMed
-
- Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 2019;381:2416–28. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

