From Acyclic Intramolecular-[4 + 2]- to Transannular Bis-[4 + 2]-Cycloaddition of the Macrodiolide for the Stereoselective Synthesis of the Octahydronaphthalene Core of Polyenic Macrolactam Sagamilactam
- PMID: 39079003
- PMCID: PMC11472481
- DOI: 10.1021/acs.orglett.4c02239
From Acyclic Intramolecular-[4 + 2]- to Transannular Bis-[4 + 2]-Cycloaddition of the Macrodiolide for the Stereoselective Synthesis of the Octahydronaphthalene Core of Polyenic Macrolactam Sagamilactam
Erratum in
-
Correction to "From Acyclic Intramolecular-[4 + 2]- to Transannular Bis-[4 + 2]-Cycloaddition of the Macrodiolide for the Stereoselective Synthesis of the Octahydronaphthalene Core of Polyenic Macrolactam Sagamilactam".Org Lett. 2024 Nov 8;26(44):9617. doi: 10.1021/acs.orglett.4c03915. Epub 2024 Oct 28. Org Lett. 2024. PMID: 39466105 Free PMC article. No abstract available.
Abstract
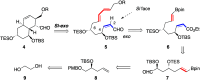
The strategy for the synthesis of the octahydronaphthalene core of natural macrolide sagamilactam has unintentionally evolved from the acyclic intramolecular (IMDA) to the transannular (TADA) Diels-Alder reaction. Lewis acid-promoted IMDA of a protected 2Z,8E,10E-4,6,12-trihydroxy-2,8,10-decatrienal model with a diol of 4,6-anti relative configuration, as proposed by DP4+-based computational studies, afforded the cis-octahydronaphthalene diastereomer through the Re-endo approach. The 26-membered macrodiolide generated, under thermal reaction conditions, the trans-octahydronaphthalene by a double TADA reaction along the desired Si-exo orientation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Kimura T.; Iwatsuki M.; Asami Y.; Ishiyama A.; Hokari R.; Otoguro K.; Matsumoto A.; Sato N.; Shiomi K.; Takahashi Y.; O̅mura S.; Nakashima T. Anti-trypanosomal compound, sagamilactam, a new polyene macrocyclic lactam from Actinomadura sp. K13–0306. J. Antibiot. 2016, 69, 818–824. 10.1038/ja.2016.28. - DOI - PubMed
-
- Nicolaou K. C.; Snyder S. A.; Montagnon T.; Vassilikogiannakis G. The Diels-Alder Reaction in Total Synthesis. Angew. Chem., Int. Ed. 2002, 41, 1668–1698. 10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z. - DOI - PubMed
- Sara A. A.; Um-e-Farwa; Saeed A.; Kalesse M. Recent Applications of the Diels-Alder Reaction in the Synthesis of Natural Products (2017–2020). Synthesis 2022, 54, 975–998. 10.1055/a-1532-4763. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

