HIV Drug Resistance in Newly Diagnosed Young Children in the Western Cape, South Africa
- PMID: 39079031
- PMCID: PMC11408107
- DOI: 10.1097/INF.0000000000004482
HIV Drug Resistance in Newly Diagnosed Young Children in the Western Cape, South Africa
Abstract
Background: Pretreatment of HIV drug resistance among children living with HIV (CLHIV) can compromise antiretroviral therapy (ART) effectiveness. Resistance may be transmitted directly from mothers or acquired following exposure to antiretrovirals consumed through breastfeeding or administered as prophylaxis.
Methods: We performed resistance testing in children aged <3 years, newly diagnosed with HIV in Western Cape, South Africa (2021-2022), who either (1) acquired HIV via possible breastfeeding transmission from mothers who received ART (any regimen) during pregnancy/postpartum and/or (2) were exposed to protease inhibitors or integrase strand transfer inhibitors (INSTIs) in utero. Possible breastfeeding transmission was defined as testing HIV-polymerase chain reaction positive at age >28 days, after previously testing negative. We used surveillance drug-resistance mutation lists to define mutations.
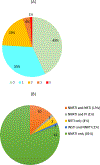
Results: We included 135 CLHIV. Most mothers started ART prepregnancy (73%). Overall, 57% (77/135) of children had resistance mutations detected. Nonnucleoside reverse transcriptase inhibitor-associated, nucleoside reverse transcriptase inhibitor-associated, protease inhibitor-associated and INSTI-associated mutations were found in 55% (74/135), 10% (13/135), <1% (1/135) and <1% (1/122) of children tested, respectively. One child with breastfeeding transmission had high-level INSTI resistance detected at HIV diagnosis, aged 18 months (E138K and G118R mutations).
Conclusions: Although not clinically relevant, nonnucleoside reverse transcriptase inhibitor-associated mutations were common. Dolutegravir is currently the preferred first-line treatment for adults and CLHIV age ≥4 weeks, and although very low INSTI resistance levels have been observed in adults, limited data exist on genotyping the integrase region in children. Pretreatment INSTI resistance in children is likely to be unusual, but future surveillance, including longitudinal studies with paired mother-child resistance testing, is needed.
Copyright © 2024 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
K.A., E.K. and M.-A.D. received funding from ViiV Healthcare for this project. The Provincial Health Data Centre was supported by grant U01AI069924 from National Institutes of Health (National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, National Cancer Institute, National Institute on Drug Abuse and National Institute of Mental Health)—Principal Investigator: M. Egger and M.-A.D. E.K., A.B. and M.-A.D. were supported by grant R01HD080465 from National Institutes of Health (National Institute of Child Health and Human Development). The other authors have no conflicts of interest to disclose.
Figures
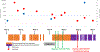
References
-
- Waitt CJ, Garner P, Bonnett LJ, Khoo SH, Else LJ. Is infant exposure to antiretroviral drugs during breastfeeding quantitatively important? A systematic review and meta-analysis of pharmacokinetic studies. J Antimicrob Chemother. 2015;70(7):1928–1941. Available from: https://academic.oup.com/jac/article/70/7/1928/777487 - PMC - PubMed
-
- Zeh C, Weidle PJ, Nafisa L, Lwamba HM, Okonji J, Anyango E, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: A secondary analysis. PLoS Med. 2011;8(3):1–10. Available from: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1... - PMC - PubMed
-
- Fogel J, Li Q, Taha TE, Hoover DR, Kumwenda NI, Mofenson LM, et al. Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV-infected infants. Clin Infect Dis. 2011;52(8):1069–76. Available from: https://academic.oup.com/cid/article/52/8/1069/286595 - PMC - PubMed
-
- Boyce CL, Sils T, Ko D, Wong-on-Wing A, Beck IA, Styrchak SM, et al. Maternal Human Immunodeficiency Virus (HIV) Drug Resistance Is Associated With Vertical Transmission and Is Prevalent in Infected Infants. Clin Infect Dis. 2022;74(11):2001–2009. Available from: https://academic.oup.com/cid/article/74/11/2001/6360962 - PMC - PubMed
-
- Dickinson L, Walimbwa S, Singh Y, Kaboggoza J, Kintu K, Sihlangu M, et al. Infant Exposure to Dolutegravir Through Placental and Breast Milk Transfer: A Population Pharmacokinetic Analysis of DolPHIN-1. Clin Infect Dis. 2021;73(5):e1200–e1207. Available from: https://academic.oup.com/cid/article/73/5/e1200/6042568 - PMC - PubMed

