Operando Raman Spectroscopy Reveals Degradation Byproducts from Ionomer Oxidation in Anion Exchange Membrane Water Electrolyzers
- PMID: 39079934
- PMCID: PMC11328119
- DOI: 10.1021/jacs.4c05721
Operando Raman Spectroscopy Reveals Degradation Byproducts from Ionomer Oxidation in Anion Exchange Membrane Water Electrolyzers
Abstract
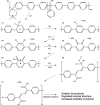
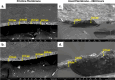
This work showcases the discovery of degradation mechanisms for nonplatinum group metal catalyst (PGM free) based anion exchange membrane water electrolyzers (AEMWE) that utilize hydroxide ion conductive polymer ionomers and membranes in a zero gap configuration. An entirely unique and customized test cell was designed from the ground up for the purposes of obtaining Raman spectra during potentiostatic operation. These results represent some of the first operando Raman spectroscopy explorations into the breakdown products that are generated from high oxidative potential conditions with carbonate electrolytes. We provide a unique design and fabrication method for three-dimensional (3D) printable flow cells that enable spatially resolved Raman spectra collection from the electrode surface into the bulk electrolyte. It is proposed that the generation of breakdown products from the hydroxide-conductive ionomers and membranes originates from a multistep, free radical reaction pathway resulting in chain scission of the poly aryl backbone. This hypothesis is backed by the detection of carboxylic and aromatic functional group Raman signals from small molecules that had dissolved and diffused into the bulk electrolyte.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


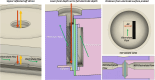
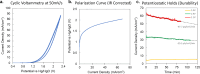

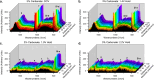
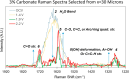

References
-
- Li D.; Motz A. R.; Bae C.; Fujimoto C.; Yang G.; Zhang F.-Y.; Ayers K. E.; Kim Y. S. Durability of Anion Exchange Membrane Water Electrolyzers. Energy Environ. Sci. 2021, 14 (6), 3393–3419. 10.1039/D0EE04086J. - DOI
-
- Simoes S. G.; Catarino J.; Picado A.; Lopes T. F.; di Berardino S.; Amorim F.; Gírio F.; Rangel C. M.; de Leão T. P. Water Availability and Water Usage Solutions for Electrolysis in Hydrogen Production. J. Cleaner Prod. 2021, 315, 12812410.1016/j.jclepro.2021.128124. - DOI
-
- Ovalle V. J.; Waegele M. M. Influence of pH and Proton Donor/Acceptor Identity on Electrocatalysis in Aqueous Media. J. Phys. Chem. C 2021, 125 (34), 18567–18578. 10.1021/acs.jpcc.1c05921. - DOI
-
- Krivina R. A.; Lindquist G. A.; Yang M. C.; Cook A. K.; Hendon C. H.; Motz A. R.; Capuano C.; Ayers K. E.; Hutchison J. E.; Boettcher S. W. Three-Electrode Study of Electrochemical Ionomer Degradation Relevant to Anion-Exchange-Membrane Water Electrolyzers. ACS Appl. Mater. Interfaces 2022, 14 (16), 18261–18274. 10.1021/acsami.1c22472. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

