Ustekinumab for type 1 diabetes in adolescents: a multicenter, double-blind, randomized phase 2 trial
- PMID: 39079992
- PMCID: PMC11405276
- DOI: 10.1038/s41591-024-03115-2
Ustekinumab for type 1 diabetes in adolescents: a multicenter, double-blind, randomized phase 2 trial
Abstract
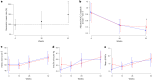
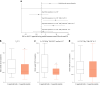
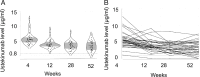
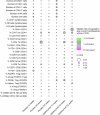
Immunotherapy targeting the autoimmune process in type 1 diabetes (T1D) can delay the loss of β-cells but needs to have minimal adverse effects to be an adjunct to insulin in the management of T1D. Ustekinumab binds to the shared p40 subunit of interleukin (IL)-12 and IL-23, targeting development of T helper 1 cells and T helper 17 cells (TH1 and TH17 cells) implicated in the pathogenesis of T1D. We conducted a double-blind, randomized controlled trial of ustekinumab in 72 adolescents aged 12-18 years with recent-onset T1D. Treatment was well tolerated with no increase in adverse events. At 12 months, β-cell function, measured by stimulated C-peptide, was 49% higher in the intervention group (P = 0.02), meeting the prespecified primary outcome. Preservation of C-peptide correlated with the reduction of T helper cells co-secreting IL-17A and interferon-γ (TH17.1 cells, P = 0.04) and, in particular, with the reduction in a subset of TH17.1 cells co-expressing IL-2 and granulocyte-macrophage colony-stimulating factor (IL-2+ GM-CSF+ TH17.1 cells, P = 0.04). A significant fall in β-cell-targeted (proinsulin-specific) IL-17A-secreting T cells was also seen (P = 0.0003). Although exploratory, our data suggest a role for an activated subset of TH17.1 cells in T1D that can be targeted with minimal adverse effects to reduce C-peptide loss, which requires confirmation in a larger study. (International Standard Randomised Controlled Trial Number Registry: ISRCTN 14274380).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Holman, N. et al. National trends in hyperglycemia and diabetic ketoacidosis in children, adolescents, and young adults with type 1 diabetes: a challenge due to age or stage of development, or is new thinking about service provision needed? Diabetes Care46, 1404–1408 (2023). 10.2337/dc23-0180 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

