Catalytic enantioselective nitrone cycloadditions enabling collective syntheses of indole alkaloids
- PMID: 39080291
- PMCID: PMC11289135
- DOI: 10.1038/s41467-024-50509-4
Catalytic enantioselective nitrone cycloadditions enabling collective syntheses of indole alkaloids
Abstract
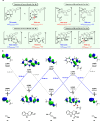
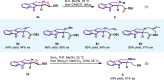
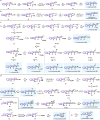
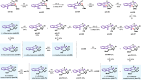
Tetrahydro-β-carboline skeletons are prominent and ubiquitous in an extraordinary range of indole alkaloid natural products and pharmaceutical compounds. Powerful synthetic approaches for stereoselective synthesis of tetrahydro-β-carboline skeletons have immense impacts and have attracted enormous attention. Here, we outline a general chiral phosphoric acid catalyzed asymmetric 1,3-dipolar cycloaddition of 3,4-dihydro-β-carboline-2-oxide type nitrone that enables access to three types of chiral tetrahydro-β-carbolines bearing continuous multi-chiral centers and quaternary chiral centers. The method displays different endo/exo selectivity from traditional nitrone chemistry. The distinct power of this strategy has been illustrated by application to collective and enantiodivergent total syntheses of 40 tetrahydro-β-carboline-type indole alkaloid natural products with divergent stereochemistry and varied architectures.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Catalytic Asymmetric Alkynylation of 3,4-Dihydro-β-carbolinium Ions Enables Collective Total Syntheses of Indole Alkaloids.Angew Chem Int Ed Engl. 2021 Nov 15;60(47):25135-25142. doi: 10.1002/anie.202112383. Epub 2021 Oct 20. Angew Chem Int Ed Engl. 2021. PMID: 34581483
-
Application of Pictet-Spengler Reaction to Indole-Based Alkaloids Containing Tetrahydro-β-carboline Scaffold in Combinatorial Chemistry.ACS Comb Sci. 2017 Apr 10;19(4):199-228. doi: 10.1021/acscombsci.6b00184. Epub 2017 Mar 17. ACS Comb Sci. 2017. PMID: 28276678 Review.
-
Chiral Pd-Catalyzed Enantioselective Syntheses of Various N-C Axially Chiral Compounds and Their Synthetic Applications.Acc Chem Res. 2021 Feb 2;54(3):719-730. doi: 10.1021/acs.accounts.0c00767. Epub 2021 Jan 22. Acc Chem Res. 2021. PMID: 33481580
-
Diastereo- and enantioselective construction of a bispirooxindole scaffold containing a tetrahydro-β-carboline moiety through an organocatalytic asymmetric cascade reaction.Chemistry. 2014 Sep 1;20(36):11382-9. doi: 10.1002/chem.201402485. Epub 2014 Jul 23. Chemistry. 2014. PMID: 25056997
-
[The chemistry of pericyclic reactions and their application to syntheses of heterocyclic compounds].Yakugaku Zasshi. 2003 Sep;123(9):717-59. doi: 10.1248/yakushi.123.717. Yakugaku Zasshi. 2003. PMID: 14513766 Review. Japanese.
Cited by
-
Regiodivergence in the Cycloadditions between a Cyclic Nitrone and Carbonyl-Type Dipolarophiles.J Org Chem. 2025 Aug 8;90(31):11020-11032. doi: 10.1021/acs.joc.5c00727. Epub 2025 Jul 29. J Org Chem. 2025. PMID: 40730878 Free PMC article.
References
-
- Maity, P., Adhikari, D. & Jana, A. K. An overview on synthetic entries to tetrahydro-β-carbolines. Tetrahedron75, 965–1028 (2019).10.1016/j.tet.2019.01.004 - DOI
-
- Glinsky-Olivier, N. & Guinchard, X. Enantioselective catalytic methods for the elaboration of chiral tetrahydro-β-carbolines and related scaffolds. Synthesis49, 2605–2620 (2017).10.1055/s-0036-1589003 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

