Structure of the MlaC-MlaD complex reveals molecular basis of periplasmic phospholipid transport
- PMID: 39080293
- PMCID: PMC11289387
- DOI: 10.1038/s41467-024-50615-3
Structure of the MlaC-MlaD complex reveals molecular basis of periplasmic phospholipid transport
Abstract
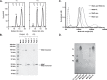
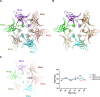
The Maintenance of Lipid Asymmetry (Mla) pathway is a multicomponent system found in all gram-negative bacteria that contributes to virulence, vesicle blebbing and preservation of the outer membrane barrier function. It acts by removing ectopic lipids from the outer leaflet of the outer membrane and returning them to the inner membrane through three proteinaceous assemblies: the MlaA-OmpC complex, situated within the outer membrane; the periplasmic phospholipid shuttle protein, MlaC; and the inner membrane ABC transporter complex, MlaFEDB, proposed to be the founding member of a structurally distinct ABC superfamily. While the function of each component is well established, how phospholipids are exchanged between components remains unknown. This stands as a major roadblock in our understanding of the function of the pathway, and in particular, the role of ATPase activity of MlaFEDB is not clear. Here, we report the structure of E. coli MlaC in complex with the MlaD hexamer in two distinct stoichiometries. Utilising in vivo complementation assays, an in vitro fluorescence-based transport assay, and molecular dynamics simulations, we confirm key residues, identifying the MlaD β6-β7 loop as essential for MlaCD function. We also provide evidence that phospholipids pass between the C-terminal helices of the MlaD hexamer to reach the central pore, providing insight into the trajectory of GPL transfer between MlaC and MlaD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
- BB/S017283/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/M01116X/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/M01116X/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/M01116X/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/S017283/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

