Potential of using an engineered indole lactic acid producing Escherichia coli Nissle 1917 in a murine model of colitis
- PMID: 39080343
- PMCID: PMC11289411
- DOI: 10.1038/s41598-024-68412-9
Potential of using an engineered indole lactic acid producing Escherichia coli Nissle 1917 in a murine model of colitis
Abstract
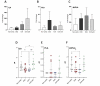
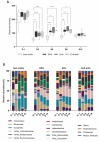
The gut microbiome is a significant factor in the pathophysiology of ulcerative colitis (UC), prompting investigations into the use of probiotic therapies to counter gastrointestinal inflammation. However, while much attention has been given to the therapeutic potential of microbes at the species and strain level, the discovery and application of their metabolic products may offer more precise and controlled solutions in battling disease. In this work, we examined the therapeutic potential of indole lactic acid (ILA) to alleviate inflammation in a murine model of colitis. A previously constructed ILA-producing Escherichia coli Nissle 1917 strain (EcN aldh) and its isogenic non-ILA producing counterpart (EcN) were studied in a murine model of Dextran Sodium Sulfate (DSS) induced colitis. The colitic animals suffered from severe colitic symptoms, with no differentiation between the groups in body weight loss and disease activity index. However, three days after cessation of DSS treatment the EcN aldh-treated mice showed signs of reduced intestinal inflammation, as manifested by lower concentrations of fecal lipocalin-2. Additionally, expression analysis of the inflamed tissue revealed distinct effects of the EcN aldh strain on proteins associated with intestinal health, such as TFF3, occludin and IL-1β expression. These results show no impact of EcN or EcN aldh on acute DSS-induced colitis, but suggest that in particular EcN aldh may assist recovery from intestinal inflammation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Genetically engineered bacteria expressing IL-34 alleviate DSS-induced experimental colitis by promoting tight junction protein expression in intestinal mucosal epithelial cells.Mol Immunol. 2025 Feb;178:64-75. doi: 10.1016/j.molimm.2025.01.008. Epub 2025 Jan 25. Mol Immunol. 2025. PMID: 39864284
-
Beneficial effects resulting from oral administration of Escherichia coli Nissle 1917 on a chronic colitis model.Benef Microbes. 2020 Dec 2;11(8):779-790. doi: 10.3920/BM2020.0045. Epub 2020 Nov 16. Benef Microbes. 2020. PMID: 33191778
-
The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice.Biochem Pharmacol. 2011 Dec 15;82(12):1891-900. doi: 10.1016/j.bcp.2011.09.004. Epub 2011 Sep 16. Biochem Pharmacol. 2011. PMID: 21930116
-
Nattokinase enhances the preventive effects of Escherichia coli Nissle 1917 on dextran sulfate sodium-induced colitis in mice.World J Microbiol Biotechnol. 2022 Nov 9;39(1):8. doi: 10.1007/s11274-022-03452-9. World J Microbiol Biotechnol. 2022. PMID: 36350434
-
[The role of Escherichia coli strain Nissle 1917 in the gastro-intestinal diseases].Postepy Hig Med Dosw (Online). 2014 Nov 6;68:1251-6. doi: 10.5604/17322693.1127882. Postepy Hig Med Dosw (Online). 2014. PMID: 25380207 Review. Polish.
Cited by
-
Buqi-Huoxue-Tongnao decoction drives gut microbiota-derived indole lactic acid to attenuate ischemic stroke via the gut-brain axis.Chin Med. 2024 Sep 15;19(1):126. doi: 10.1186/s13020-024-00991-1. Chin Med. 2024. PMID: 39278929 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

