Predictive value of inflammation and nutritional index in immunotherapy for stage IV non-small cell lung cancer and model construction
- PMID: 39080372
- PMCID: PMC11289435
- DOI: 10.1038/s41598-024-66813-4
Predictive value of inflammation and nutritional index in immunotherapy for stage IV non-small cell lung cancer and model construction
Erratum in
-
Author Correction: Predictive value of inflammation and nutritional index in immunotherapy for stage IV non-small cell lung cancer and model construction.Sci Rep. 2024 Aug 22;14(1):19518. doi: 10.1038/s41598-024-70611-3. Sci Rep. 2024. PMID: 39174740 Free PMC article. No abstract available.
Abstract
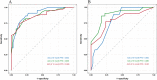
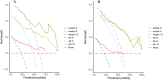
Identifying individuals poised to gain from immune checkpoint inhibitor (ICI) therapies is a pivotal element in the realm of tailored healthcare. The expression level of Programmed Death Ligand 1 (PD-L1) has been linked to the response to ICI therapy, but its assessment typically requires substantial tumor tissue, which can be challenging to obtain. In contrast, blood samples are more feasible for clinical application. A number of promising peripheral biomarkers have been proposed to overcome this hurdle. This research aims to evaluate the prognostic utility of the albumin-to-lactate dehydrogenase ratio (LAR), the Pan-immune-inflammation Value (PIV), and the Prognostic Nutritional Index (PNI) in predicting the response to ICI therapy in individuals with advanced non-small cell lung cancer (NSCLC). Furthermore, the study seeks to construct a predictive nomogram that includes these markers to facilitate the selection of patients with a higher likelihood of benefiting from ICI therapy. A research initiative scrutinized the treatment records of 157 advanced NSCLC patients who received ICI therapy across two Jiangxi medical centers. The cohort from Jiangxi Provincial People's Hospital (comprising 108 patients) was utilized for the training dataset, while the contingent from Jiangxi Cancer Hospital (49 patients) served for validation purposes. Stratification was based on established LAR, PIV, and PNI benchmarks to explore associations with DCR and ORR metrics. Factorial influences on ICI treatment success were discerned through univariate and multivariate Cox regression analysis. Subsequently, a Nomogram was devised to forecast outcomes, its precision gauged by ROC and calibration curves, DCA analysis, and cross-institutional validation. In the training group, the optimal threshold values for LAR, PIV, and PNI were identified as 5.205, 297.49, and 44.6, respectively. Based on these thresholds, LAR, PIV, and PNI were categorized into high (≥ Cut-off) and low (< Cut-off) groups. Patients with low LAR (L-LAR), low PIV (L-PIV), and high PNI (H-PNI) exhibited a higher disease control rate (DCR) (P < 0.05) and longer median progression-free survival (PFS) (P < 0.05). Cox multivariate analysis indicated that PS, malignant pleural effusion, liver metastasis, high PIV (H-PIV), and low PNI (L-PNI) were risk factors adversely affecting the efficacy of immunotherapy (P < 0.05). The Nomogram model predicted a concordance index (C-index) of 0.78 (95% CI: 0.73-0.84). The areas under the ROC curve (AUC) for the training group at 6, 9, and 12 months were 0.900, 0.869, and 0.866, respectively, while the AUCs for the external validation group at the same time points were 0.800, 0.886, and 0.801, respectively. Throughout immunotherapy, PIV and PNI could act as prospective indicators for forecasting treatment success in NSCLC patients, while the devised Nomogram model exhibits strong predictive performance for patient prognoses.
Keywords: Immunotherapy; LAR; NSCLC; Nomogram model; PIV; PNI.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Construction of a prognostic model for extensive-stage small cell lung cancer patients undergoing immune therapy in northernmost China and prediction of treatment efficacy based on response status at different time points.J Cancer Res Clin Oncol. 2024 May 15;150(5):255. doi: 10.1007/s00432-024-05767-6. J Cancer Res Clin Oncol. 2024. PMID: 38750370 Free PMC article.
-
Efficacy and safety of immunotherapy in real-world patients with advanced non-small cell lung cancer.Cancer Treat Res Commun. 2025;43:100908. doi: 10.1016/j.ctarc.2025.100908. Epub 2025 Mar 24. Cancer Treat Res Commun. 2025. PMID: 40187204
-
The systemic inflammation response index (SIRI) predicts survival in advanced non-small cell lung cancer patients undergoing immunotherapy and the construction of a nomogram model.Front Immunol. 2024 Dec 24;15:1516737. doi: 10.3389/fimmu.2024.1516737. eCollection 2024. Front Immunol. 2024. PMID: 39776905 Free PMC article.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
The landscape of immune checkpoint inhibitor plus chemotherapy versus immunotherapy for advanced non-small-cell lung cancer: A systematic review and meta-analysis.J Cell Physiol. 2020 May;235(5):4913-4927. doi: 10.1002/jcp.29371. Epub 2019 Nov 6. J Cell Physiol. 2020. PMID: 31693178 Free PMC article.
Cited by
-
The Prognostic Significance of the CALLY Index in Ampullary Carcinoma: An Inflammation-Nutrition Retrospective Analysis.J Inflamm Res. 2025 Jan 16;18:621-635. doi: 10.2147/JIR.S495815. eCollection 2025. J Inflamm Res. 2025. PMID: 39835294 Free PMC article.
-
The value of prognostic immune-inflammatory-nutritional score in predicting survival after surgery in stage I-III non-small cell lung cancer.J Thorac Dis. 2025 Jun 30;17(6):3886-3896. doi: 10.21037/jtd-24-1830. Epub 2025 Jun 23. J Thorac Dis. 2025. PMID: 40688309 Free PMC article.
-
Prognostic value of the lactate dehydrogenase to albumin ratio in cancer patients.Front Nutr. 2025 Jul 7;12:1610487. doi: 10.3389/fnut.2025.1610487. eCollection 2025. Front Nutr. 2025. PMID: 40693202 Free PMC article.
-
Pan-immune-inflammation value and its association with all-cause and cause-specific mortality in the general population: a nationwide cohort study.Front Endocrinol (Lausanne). 2025 Apr 30;16:1534018. doi: 10.3389/fendo.2025.1534018. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40370772 Free PMC article.
-
Prognostic scoring system using inflammation- and nutrition-related biomarkers to predict prognosis in stage I-III colorectal cancer patients.World J Gastroenterol. 2025 Apr 14;31(14):104588. doi: 10.3748/wjg.v31.i14.104588. World J Gastroenterol. 2025. PMID: 40248373 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

