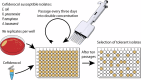
In vitro resistance development gives insights into molecular resistance mechanisms against cefiderocol
- PMID: 39080477
- PMCID: PMC11513634
- DOI: 10.1038/s41429-024-00762-y
In vitro resistance development gives insights into molecular resistance mechanisms against cefiderocol
Abstract
Cefiderocol, a novel siderophore cephalosporin, demonstrates promising in vitro activity against multidrug-resistant Gram-negative bacteria, including carbapenemase-producing strains. Nonetheless, only a few reports are available regarding the acquisition of resistance in clinical settings, primarily due to its recent usage. This study aimed to investigate cefiderocol resistance using an in vitro resistance development model to gain insights into the underlying molecular resistance mechanisms. Cefiderocol susceptible reference strains (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa) and a clinical Acinetobacter baumannii complex isolate were exposed to increasing cefiderocol concentrations using a high-throughput resistance development model. Cefiderocol susceptibility testing was performed using broth microdilution. Whole-genome sequencing was employed to identify newly acquired resistance mutations. Our in vitro resistance development model led to several clones of strains exhibiting cefiderocol resistance, with MIC values 8-fold to 512-fold higher than initial levels. In total, we found 42 different mutations in 26 genes, of which 35 could be described for the first time. Putative loss-of-function mutations were detected in the envZ, tonB, and cirA genes in 13 out of 17 isolates, leading to a decrease in cefiderocol influx. Other potential resistance mechanisms included multidrug efflux pumps (baeS, czcS, nalC), antibiotic-inactivating enzymes (ampR, dacB), and target mutations in penicillin-binding-protein genes (mrcB). This study reveals new insights into underlying molecular resistance mechanisms against cefiderocol. While mutations leading to reduced influx via iron transporters was the most frequent resistance mechanism, we also detected several other novel resistance mutations causing cefiderocol resistance.
© 2024. The Author(s).
Conflict of interest statement
FL received speaker fees from Shionogi. BW received speaker fees and project funding allocated to a different project from Shionogi. MZ acted as member of advisory boards for Shionogi and his department received funding for independent research from Shionogi. All other authors: none to declare.
Figures
Similar articles
-
Susceptibility toward cefiderocol and sulbactam-durlobactam in extensively drug-resistant Acinetobacter baumannii detected from ICU admission screening in Hanoi, Vietnam, 2023.Microbiol Spectr. 2025 Jul;13(7):e0083225. doi: 10.1128/spectrum.00832-25. Epub 2025 Jun 9. Microbiol Spectr. 2025. PMID: 40488459 Free PMC article.
-
Spanish nationwide survey of Pseudomonas aeruginosa cefiderocol susceptibility and resistance mechanisms.Int J Antimicrob Agents. 2025 Oct;66(4):107563. doi: 10.1016/j.ijantimicag.2025.107563. Epub 2025 Jul 1. Int J Antimicrob Agents. 2025. PMID: 40609707
-
In Vitro Evolution of Cefiderocol Resistance in an NDM-Producing Klebsiella pneumoniae Due to Functional Loss of CirA.Microbiol Spectr. 2021 Dec 22;9(3):e0177921. doi: 10.1128/Spectrum.01779-21. Epub 2021 Nov 10. Microbiol Spectr. 2021. PMID: 34756080 Free PMC article.
-
Global prevalence of cefiderocol non-susceptibility in Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia: a systematic review and meta-analysis.Clin Microbiol Infect. 2024 Feb;30(2):178-188. doi: 10.1016/j.cmi.2023.08.029. Epub 2023 Sep 4. Clin Microbiol Infect. 2024. PMID: 37666449
-
Safety and efficacy of cefiderocol for off-label treatment indications: A systematic review.Pharmacotherapy. 2022 Jul;42(7):549-566. doi: 10.1002/phar.2704. Epub 2022 Jun 4. Pharmacotherapy. 2022. PMID: 35611627
Cited by
-
Import of global high-risk clones is the primary driver of carbapenemase-producing Pseudomonas aeruginosa in Norway.J Med Microbiol. 2025 Jan;74(1):001944. doi: 10.1099/jmm.0.001944. J Med Microbiol. 2025. PMID: 39760484 Free PMC article.
-
High-level cefiderocol and ceftazidime/avibactam resistance in KPC-producing Klebsiella pneumoniae associated with mutations in KPC and the sensor histidine kinase EnvZ.J Antimicrob Chemother. 2025 Apr 2;80(4):1155-1157. doi: 10.1093/jac/dkaf048. J Antimicrob Chemother. 2025. PMID: 39969122 Free PMC article. No abstract available.
-
Potential Use of Cefiderocol and Nanosilver in Wound Dressings to Control Multidrug-Resistant Gram-Negative Bacteria.Molecules. 2025 Jul 23;30(15):3072. doi: 10.3390/molecules30153072. Molecules. 2025. PMID: 40807247 Free PMC article.
-
In vitro activity of cefiderocol against Gram-negative aerobic bacilli in planktonic and biofilm form-alone and in combination with bacteriophages.Sci Rep. 2025 May 16;15(1):17105. doi: 10.1038/s41598-025-01704-w. Sci Rep. 2025. PMID: 40379736 Free PMC article.
-
Exploring mutational possibilities of KPC variants to reach high level resistance to cefiderocol.Sci Rep. 2025 Aug 25;15(1):31312. doi: 10.1038/s41598-025-17044-8. Sci Rep. 2025. PMID: 40855208 Free PMC article.
References
-
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. - PMC - PubMed
-
- European Centre for Disease Prevention and Control., World Health Organization. Antimicrobial resistance surveillance in Europe 2023: 2021 data. LU: Publications Office; 2023. Available from: https://data.europa.eu/doi/10.2900/63495. - DOI
-
- WHO. Antibacterial agents in clinical and preclinical development: an overview and analysis. 2021. Available from: https://www.who.int/publications/i/item/9789240047655.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases