A unique serum IgG glycosylation signature predicts development of Crohn's disease and is associated with pathogenic antibodies to mannose glycan
- PMID: 39080486
- PMCID: PMC11362009
- DOI: 10.1038/s41590-024-01916-8
A unique serum IgG glycosylation signature predicts development of Crohn's disease and is associated with pathogenic antibodies to mannose glycan
Abstract
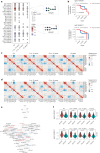
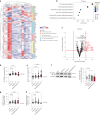
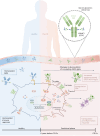
Inflammatory bowel disease (IBD) is characterized by chronic inflammation in the gut. There is growing evidence in Crohn's disease (CD) of the existence of a preclinical period characterized by immunological changes preceding symptom onset that starts years before diagnosis. Gaining insight into this preclinical phase will allow disease prediction and prevention. Analysis of preclinical serum samples, up to 6 years before IBD diagnosis (from the PREDICTS cohort), revealed the identification of a unique glycosylation signature on circulating antibodies (IgGs) characterized by lower galactosylation levels of the IgG fragment crystallizable (Fc) domain that remained stable until disease diagnosis. This specific IgG2 Fc glycan trait correlated with increased levels of antimicrobial antibodies, specifically anti-Saccharomyces cerevisiae (ASCA), pinpointing a glycome-ASCA hub detected in serum that predates by years the development of CD. Mechanistically, we demonstrated that this agalactosylated glycoform of ASCA IgG, detected in the preclinical phase, elicits a proinflammatory immune pathway through the activation and reprogramming of innate immune cells, such as dendritic cells and natural killer cells, via an FcγR-dependent mechanism, triggering NF-κB and CARD9 signaling and leading to inflammasome activation. This proinflammatory role of ASCA was demonstrated to be dependent on mannose glycan recognition and galactosylation levels in the IgG Fc domain. The pathogenic properties of (anti-mannose) ASCA IgG were validated in vivo. Adoptive transfer of antibodies to mannan (ASCA) to recipient wild-type mice resulted in increased susceptibility to intestinal inflammation that was recovered in recipient FcγR-deficient mice. Here we identify a glycosylation signature in circulating IgGs that precedes CD onset and pinpoint a specific glycome-ASCA pathway as a central player in the initiation of inflammation many years before CD diagnosis. This pathogenic glyco-hub may constitute a promising new serum biomarker for CD prediction and a potential target for disease prevention.
© 2024. The Author(s).
Conflict of interest statement
G.L. is the founder and owner of Genos, Ltd., a private research organization that specializes in high-throughput glycomic analysis, and has several patents in this field. M.N. and J.Š. are employees of Genos, Ltd. The other authors declare no competing interests.
Figures


References
-
- Torres, J. et al. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology149, 1716–1730 (2015). - PubMed
-
- Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature448, 427–434 (2007). - PubMed
-
- Morão, B. & Torres, J. Preclinical disease and preventive strategies in IBD. Curr. Opin. Pharmacol.55, 110–115 (2020). - PubMed
-
- Torres, J., Burisch, J., Riddle, M., Dubinsky, M. & Colombel, J.-F. Preclinical disease and preventive strategies in IBD: perspectives, challenges and opportunities. Gut65, 1061–1069 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

