Refined tamoxifen administration in mice by encouraging voluntary consumption of palatable formulations
- PMID: 39080504
- PMCID: PMC11291282
- DOI: 10.1038/s41684-024-01409-z
Refined tamoxifen administration in mice by encouraging voluntary consumption of palatable formulations
Abstract
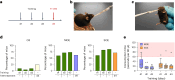
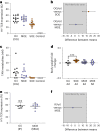
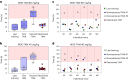
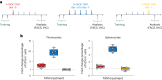
Drug administration in preclinical rodent models is essential for research and the development of novel therapies. Compassionate administration methods have been developed, but these are mostly incompatible with water-insoluble drugs such as tamoxifen or do not allow for precise timing or dosing of the drugs. For more than two decades, tamoxifen has been administered by oral gavage or injection to CreERT2-loxP gene-modified mouse models to spatiotemporally control gene expression, with the numbers of such inducible models steadily increasing in recent years. Animal-friendly procedures for accurately administering tamoxifen or other water-insoluble drugs would, therefore, have an important impact on animal welfare. On the basis of a previously published micropipette feeding protocol, we developed palatable formulations to encourage voluntary consumption of tamoxifen. We evaluated the acceptance of the new formulations by mice during training and treatment and assessed the efficacy of tamoxifen-mediated induction of CreERT2-loxP-dependent reporter genes. Both sweetened milk and syrup-based formulations encouraged mice to consume tamoxifen voluntarily, but only sweetened milk formulations were statistically noninferior to oral gavage or intraperitoneal injections in inducing CreERT2-mediated gene expression. Serum concentrations of tamoxifen metabolites, quantified using an in-house-developed cell assay, confirmed the lower efficacy of syrup- as compared to sweetened milk-based formulations. We found dosing with a micropipette to be more accurate than oral gavage or injection, with the added advantage that the method requires little training for the experimenter. The new palatable solutions encourage voluntary consumption of tamoxifen without loss of efficacy compared to oral gavage or injections and thus represent a refined administration method.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Sex and strain differences in the micropipette-guided drug administration (MDA) method in mice.Lab Anim. 2025 Jun 3:236772251320843. doi: 10.1177/00236772251320843. Online ahead of print. Lab Anim. 2025. PMID: 40457987
-
Validation of a refined protocol for mouse oral glucose tolerance testing without gavage.bioRxiv [Preprint]. 2024 Sep 19:2024.09.13.612859. doi: 10.1101/2024.09.13.612859. bioRxiv. 2024. PMID: 39345490 Free PMC article. Preprint.
-
A shortened tamoxifen induction scheme to induce CreER recombinase without side effects on the male mouse skeleton.Mol Cell Endocrinol. 2017 Sep 5;452:57-63. doi: 10.1016/j.mce.2017.05.012. Epub 2017 May 11. Mol Cell Endocrinol. 2017. PMID: 28504114
-
Temporally controlled somatic mutagenesis in smooth muscle.Genesis. 2000 Sep;28(1):15-22. doi: 10.1002/1526-968x(200009)28:1<15::aid-gene20>3.0.co;2-c. Genesis. 2000. PMID: 11020712
-
Oral and Injected Tamoxifen Alter Adult Hippocampal Neurogenesis in Female and Male Mice.eNeuro. 2022 Apr 20;9(2):ENEURO.0422-21.2022. doi: 10.1523/ENEURO.0422-21.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35387845 Free PMC article.
References
-
- Russel, W. M. S. & Burch, R. L. The Principles of Humane Experimental Technique (Methuen & Co., 1959).
MeSH terms
Substances
Grants and funding
- BU1410/1-2/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 310030_132713 / 1/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 310030_116201/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
LinkOut - more resources
Full Text Sources

