A novel dual-target Septin9 methylation assay for improved detection of early-stage colorectal cancer and high-grade intraepithelial neoplasia
- PMID: 39080571
- PMCID: PMC11290180
- DOI: 10.1186/s12885-024-12645-4
A novel dual-target Septin9 methylation assay for improved detection of early-stage colorectal cancer and high-grade intraepithelial neoplasia
Abstract
Background: Colorectal cancer (CRC) ranks as the third most common malignancies in the world, and periodic examination of the patient is advantageous in reducing the mortality of CRC. The first blood-based Septin9 gene methylation assay which recognized by the US FDA for CRC examination was Epi proColon. However, this assay was not broadly applied in the current clinical guideline because of its relatively lower sensitivity in the detection of early-stage CRC.
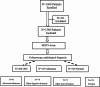
Methods: This study aimed at developing a new multiplex Septin9 methylation assay (ColonUSK) which simultaneously evaluates two CpG-rich subregions in the promoter of the Septin9 gene and an internal control in a single reaction. ColonUSK proved increased sensitivity, with a detection limit as low as 12pg of the positive DNA compared with the Septin9 assay targeting one CpG-rich subregion. 1366 subjects were prospectively recruited from four comprehensive hospitals in China in an opportunistic screening study for assessing its value in CRC detection. Blind testing was developed to evaluate ColonUSK in comparison with clinical examination using clinical gold standard such as colonoscopy.
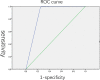
Results: The assay demonstrates clinical sensitivity for diagnosing colorectal cancer (CRC) and advanced adenoma at rates of 77.34% and 25.26%, respectively. Furthermore, ColonUSK exhibits a high degree of specificity for non-CRC cases (95.95%) clinically. Significantly, the detection rate of cases in high-grade intraepithelial neoplasia increased to 54.29%. The value for the assay in the Kappa test was 0.76, showing a high degree of consistency between ColonUSK and clinical gold standard.
Conclusions: ColonUSK indicated moderate diagnostic value and could become a non-invasive detection way for CRC. The implementation of the ColonUSK assay has the capacity to markedly enhance CRC screening practices.
Keywords: Colorectal cancer (CRC); CpG-rich subregions; Methylation; PCR; Septin9.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Methylation of Septin9, SRSF1, and PAX8 in Early Screening of Colorectal Cancer in the Population Undergoing Physical Examinations.Clin Lab. 2023 Dec 1;69(12). doi: 10.7754/Clin.Lab.2023.230426. Clin Lab. 2023. PMID: 38084698
-
Plasma Septin9 versus fecal immunochemical testing for colorectal cancer screening: a prospective multicenter study.PLoS One. 2014 Jun 5;9(6):e98238. doi: 10.1371/journal.pone.0098238. eCollection 2014. PLoS One. 2014. PMID: 24901436 Free PMC article.
-
Evaluation of Multigene Methylation for Blood-Based Detection of Colorectal Cancer.Genet Test Mol Biomarkers. 2024 Oct;28(10):402-409. doi: 10.1089/gtmb.2023.0754. Epub 2024 Sep 23. Genet Test Mol Biomarkers. 2024. PMID: 39308406
-
Diagnostic Value and Clinical Significance of Methylated SEPT9 for Colorectal Cancer: A Meta-Analysis.Med Sci Monit. 2019 Aug 5;25:5813-5822. doi: 10.12659/MSM.915472. Med Sci Monit. 2019. PMID: 31378778 Free PMC article.
-
SEPT9: A Specific Circulating Biomarker for Colorectal Cancer.Adv Clin Chem. 2015;72:171-204. doi: 10.1016/bs.acc.2015.07.004. Epub 2015 Aug 29. Adv Clin Chem. 2015. PMID: 26471083 Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin 2015, 65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical