Cholinergic interneurons in the nucleus accumbens are a site of cellular convergence for corticotropin-releasing factor and estrogen regulation in male and female mice
- PMID: 39080914
- PMCID: PMC12348452
- DOI: 10.1111/ejn.16477
Cholinergic interneurons in the nucleus accumbens are a site of cellular convergence for corticotropin-releasing factor and estrogen regulation in male and female mice
Abstract
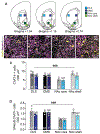
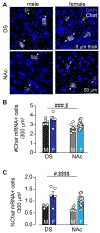
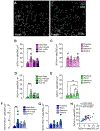
Cholinergic interneurons (ChIs) act as master regulators of striatal output, finely tuning neurotransmission to control motivated behaviours. ChIs are a cellular target of many peptide and hormonal neuromodulators, including corticotropin-releasing factor, opioids, insulin and leptin, which can influence an animal's behaviour by signalling stress, pleasure, pain and nutritional status. However, little is known about how sex hormones via estrogen receptors influence the function of these other neuromodulators. Here, we performed in situ hybridisation on mouse striatal tissue to characterise the effect of sex and sex hormones on choline acetyltransferase (Chat), estrogen receptor alpha (Esr1) and corticotropin-releasing factor type 1 receptor (Crhr1) expression. Although we did not detect sex differences in ChAT protein levels in the dorsal striatum or nucleus accumbens, we found that female mice have more Chat mRNA-expressing neurons than males in both the dorsal striatum and nucleus accumbens. At the population level, we observed a sexually dimorphic distribution of Esr1- and Crhr1-expressing ChIs in the ventral striatum that was negatively correlated in intact females, which was abolished by ovariectomy and not present in males. Only in the NAc did we find a significant population of ChIs that co-express Crhr1 and Esr1 in females and to a lesser extent in males. At the cellular level, Crhr1 and Esr1 transcript levels were negatively correlated only during the estrus phase in females, indicating that changes in sex hormone levels can modulate the interaction between Crhr1 and Esr1 mRNA levels.
Keywords: cholinergic interneuron; corticotropin releasing factor; estrogen receptors; nucleus accumbens.
© 2024 The Author(s). European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures


Update of
-
Cholinergic interneurons in the nucleus accumbens are a site of cellular convergence for corticotropin release factor and estrogen regulation.bioRxiv [Preprint]. 2024 Apr 15:2024.04.13.589360. doi: 10.1101/2024.04.13.589360. bioRxiv. 2024. Update in: Eur J Neurosci. 2024 Sep;60(5):4937-4953. doi: 10.1111/ejn.16477. PMID: 38659848 Free PMC article. Updated. Preprint.
Similar articles
-
Cholinergic interneurons in the nucleus accumbens are a site of cellular convergence for corticotropin release factor and estrogen regulation.bioRxiv [Preprint]. 2024 Apr 15:2024.04.13.589360. doi: 10.1101/2024.04.13.589360. bioRxiv. 2024. Update in: Eur J Neurosci. 2024 Sep;60(5):4937-4953. doi: 10.1111/ejn.16477. PMID: 38659848 Free PMC article. Updated. Preprint.
-
Projections from ventral hippocampus to nucleus accumbens' cholinergic neurons are altered in depression.J Gen Physiol. 2025 May 5;157(3):e202413693. doi: 10.1085/jgp.202413693. Epub 2025 Mar 7. J Gen Physiol. 2025. PMID: 40052940
-
Increased CRF-R1 transmission in the nucleus accumbens shell facilitates maternal neglect in lactating rats and mediates anxiety-like behaviour in a sex-specific manner.Neuropharmacology. 2025 Mar 1;265:110256. doi: 10.1016/j.neuropharm.2024.110256. Epub 2024 Dec 6. Neuropharmacology. 2025. PMID: 39647775
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Pharmacological interventions for those who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2015 Feb 18;2015(2):CD007989. doi: 10.1002/14651858.CD007989.pub2. Cochrane Database Syst Rev. 2015. PMID: 25692326 Free PMC article.
Cited by
-
Sex and estradiol effects in the rodent dorsal striatum.Eur J Neurosci. 2024 Dec;60(12):6962-6986. doi: 10.1111/ejn.16607. Epub 2024 Nov 21. Eur J Neurosci. 2024. PMID: 39573926 Free PMC article. Review.
-
Estrogen regulation of the nucleus accumbens as a gateway to understanding menopause associated metabolic dysfunction.NPJ Womens Health. 2025;3:24. doi: 10.1038/s44294-025-00071-1. Epub 2025 Apr 23. NPJ Womens Health. 2025. PMID: 40874093 Free PMC article.
-
Nicotine is an Immunosuppressant: Implications for Women's Health and Disease.J Neuroimmunol. 2024 Dec 15;397:578468. doi: 10.1016/j.jneuroim.2024.578468. Epub 2024 Oct 20. J Neuroimmunol. 2024. PMID: 39461120 Review.
-
Extrinsic and intrinsic control of striatal cholinergic interneuron activity.Front Mol Neurosci. 2025 Feb 13;18:1528419. doi: 10.3389/fnmol.2025.1528419. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40018010 Free PMC article. Review.
References
-
- Aoki M, Shimozuru M, Kikusui T, Takeuchi Y, Mori Y, 2010. Sex differences in behavioral and corticosterone responses to mild stressors in ICR mice are altered by ovariectomy in peripubertal period. Zoolog Sci 27, 783–789. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

