Multiplex plasma protein assays as a diagnostic tool for lung cancer
- PMID: 39080998
- PMCID: PMC11447887
- DOI: 10.1111/cas.16300
Multiplex plasma protein assays as a diagnostic tool for lung cancer
Abstract
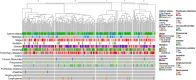
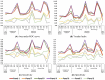
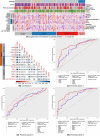
Lack of the established noninvasive diagnostic biomarkers causes delay in diagnosis of lung cancer (LC). The aim of this study was to explore the association between inflammatory and cancer-associated plasma proteins and LC and thereby discover potential biomarkers. Patients referred for suspected LC and later diagnosed with primary LC, other cancers, or no cancer (NC) were included in this study. Demographic information and plasma samples were collected, and diagnostic information was later retrieved from medical records. Relative quantification of 92 plasma proteins was carried out using the Olink Immuno-Onc-I panel. Association between expression levels of panel of proteins with different diagnoses was assessed using generalized linear model (GLM) with the binomial family and a logit-link function, considering confounder effects of age, gender, smoking, and pulmonary diseases. The analysis showed that the combination of five plasma proteins (CD83, GZMA, GZMB, CD8A, and MMP12) has higher diagnostic performance for primary LC in both early and advanced stages compared with NC. This panel demonstrated lower diagnostic performance for other cancer types. Moreover, inclusion of four proteins (GAL9, PDCD1, CD4, and HO1) to the aforementioned panel significantly increased the diagnostic performance for primary LC in advanced stage as well as for other cancers. Consequently, the collective expression profiles of select plasma proteins, especially when analyzed in conjunction, might have the potential to distinguish individuals with LC from NC. This suggests their utility as predictive biomarkers for identification of LC patients. The synergistic application of these proteins as biomarkers could pave the way for the development of diagnostic tools for early-stage LC detection.
Keywords: confounder correction; diagnostic biomarker; liquid biopsy; lung cancer; machine learning; plasma proteomics; screening.
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflict of interest related to this study.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39‐51. - PubMed
-
- Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193‐200. - PubMed
-
- Shevchenko VE, Arnotskaya NE, Zaridze DG. Detection of lung cancer using plasma protein profiling by matrix‐assisted laser desorption/ionization mass spectrometry. Eur J Mass Spectrom (Chichester). 2010;16(4):539‐549. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

