Blockade of CaV3 calcium channels and induction of G0/G1 cell cycle arrest in colon cancer cells by gossypol
- PMID: 39081110
- PMCID: PMC11613961
- DOI: 10.1111/bph.16497
Blockade of CaV3 calcium channels and induction of G0/G1 cell cycle arrest in colon cancer cells by gossypol
Abstract
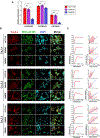
Background and purpose: Gastrointestinal tumours overexpress voltage-gated calcium (CaV3) channels (CaV3.1, 3.2 and 3.3). CaV3 channels regulate cell growth and apoptosis colorectal cancer. Gossypol, a polyphenolic aldehyde found in the cotton plant, has anti-tumour properties and inhibits CaV3 currents. A systematic study was performed on gossypol blocking mechanism on CaV3 channels and its potential anticancer effects in colon cancer cells, which express CaV3 isoforms.
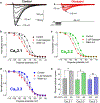
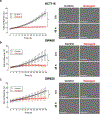
Experimental approach: Transcripts for CaV3 proteins were analysed in gastrointestinal cancers using public repositories and in human colorectal cancer cell lines HCT116, SW480 and SW620. The gossypol blocking mechanism on CaV3 channels was investigated by combining heterologous expression systems and patch-clamp experiments. The anti-tumoural properties of gossypol were estimated by cell proliferation, viability and cell cycle assays. Ca2+ dynamics were evaluated with cytosolic and endoplasmic reticulum (ER) Ca2+ indicators.
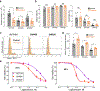
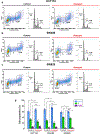
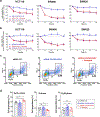
Key results: High levels of CaV3 transcripts correlate with poor prognosis in gastrointestinal cancers. Gossypol blockade of CaV3 isoforms is concentration- and use-dependent interacting with the closed, activated and inactivated conformations of CaV3 channels. Gossypol and CaV3 channels down-regulation inhibit colorectal cancer cell proliferation by arresting cell cycles at the G0/G1 and G2/M phases, respectively. CaV3 channels underlie the vectorial Ca2+ uptake by endoplasmic reticulum in colorectal cancer cells.
Conclusion and implications: Gossypol differentially blocked CaV3 channel and its anticancer activity was correlated with high levels of CaV3.1 and CaV3.2 in colorectal cancer cells. The CaV3 regulates cell proliferation and Ca2+ dynamics in colorectal cancer cells. Understanding this blocking mechanism maybe improve cancer therapies.
Keywords: CaV3 calcium channels; TTA‐P2; blocking mechanism; gastrointestinal cancers; gossypol; ion channel blockers.
© 2024 The Author(s). British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
Figures




References
-
- Alexander SPH, Fabbro D, Kelly E, Mathie AA, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Davies JA, Annett S, Boison D, Burns KE, Dessauer C, Gertsch J, Helsby NA, Izzo AA, Ostrom R, Papapetropoulos A, … Wong SS (2023). The Concise Guide to PHARMACOLOGY 2023/24: Enzymes. British Journal of Pharmacology, 180(S2). 10.1111/bph.16181 - DOI - PubMed
-
- Alexander SPH, Mathie AA, Peters JA, Veale EL, Striessnig J, Kelly E, Armstrong JF, Faccenda E, Harding SD, Davies JA, Aldrich RW, Attali B, Baggetta AM, Becirovic E, Biel M, Bill RM, Caceres AI, Catterall WA, Conner AC, … Zhu M (2023). The Concise Guide to PHARMACOLOGY 2023/24: Ion channels. British Journal of Pharmacology, 180(S2). 10.1111/bph.16178 - DOI - PubMed
-
- Alexander SPH, Roberts RE, Broughton BRS, Sobey CG, George CH, Stanford SC, Cirino G, Docherty JR, Giembycz MA, Hoyer D, Insel PA, Izzo AA, Ji Y, MacEwan DJ, Mangum J, Wonnacott S, & Ahluwalia A (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- SPF201909009198/Fondation pour la Recherche Médicale (FRM), France
- BB/C517317/1/Biotechnology and Biological Sciences Research Council, UK
- G2022026006L/National High-End Foreign Expert Recruitment Plan of China, China
- pre-R01grant/O'Neal Comprehensive Cancer Center, USA
- CVU1148606/Consejo Nacional de Ciencia y Tecnologia (CONACYT), Mexico
- PrixRubanRoseAvenir/Le Cancer du sein, parlons-en, France
- 16IRTSTHN020/Department of Education of the Henan Province, China
- Ministère de la Recherche et des Technologies, France
- Université de Tours, France
- IN209820/PAPIIT-DGAPA-UNAM, Mexico
- NavMetarget/Conseil Régional du Centre-Val de Loire, France
- 1R21CA226491/National Institutes of Health (NIH), USA
- R21 CA226491/CA/NCI NIH HHS/United States
- 099758/Z/12/Z/Wellcome Trust, UK
- CanalEx/Conseil Régional du Centre-Val de Loire, France
- I1200/320/2022 CVU 369878/Consejo Nacional de Ciencia y Tecnologia (CONACYT), Mexico
- Ligue Nationale Contre le Cancer, Interrégion Grand-Ouest: comités 29, 36, 86 and 37, France
- 2016PN-KFKT-06/Disciplinary Group of Psychology and Neuroscience, Xinxiang Medical University, China
- WT_/Wellcome Trust/United Kingdom
- A1-S-19171/Consejo Nacional de Ciencia y Tecnologia (CONACYT), Mexico
LinkOut - more resources
Full Text Sources
Miscellaneous

