Key insights and challeneges in noninferiority trials
- PMID: 39081188
- PMCID: PMC11294879
- DOI: 10.4097/kja.23534
Key insights and challeneges in noninferiority trials
Abstract
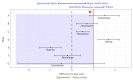
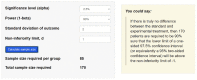
Noninferiority clinical trials are crucial for evaluating the effectiveness of new interventions compared to standard interventions. By establishing statistical and clinical comparability, these trials can be conducted to demonstrate that a new intervention is not significantly inferior to the standard intervention. However, selecting appropriate noninferiority margins and study designs are essential to ensuring valid and reliable results. Moreover, employing the Consolidated Standards of Reporting Trials (CONSORT) statement for reporting noninferiority clinical trials enhances the quality and transparency of research findings. This article addresses key considerations and challenges faced by investigators in planning, conducting, and interpreting the results of noninferiority clinical trials.
Keywords: Bias; Clinical trial; Noninferiority; Randomized controlled trial; Research design; Sample size; Treatment outcome..
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Kim HJ, Koh KH, Park JI, Kim YJ, Kim MJ, Kim H, et al. Comparison of the analgesic efficacy between arthroscopically placed continuous suprascapular nerve block and ultrasound-guided continuous superior trunk block: a double-blinded randomized controlled trial. Anesthesiology. 2023;139:591–601. - PubMed
-
- Kwon HJ, Lee JB, Lee K, Shin JY, Jeong SM, Lee JH, et al. Real-time ultrasound guidance versus fluoroscopic guidance in thoracic epidural catheter placement: a single-center, non-inferiority, randomized, active-controlled trial. Reg Anesth Pain Med. 2024;49:168–73. - PubMed
-
- Lee S, Kang RA, Kim GS, Gwak MS, Choi GS, Kim JM, et al. Comparison of postoperative analgesic effects of posterior quadratus lumborum block and intrathecal morphine in laparoscopic donor hepatectomy: a prospective randomized non-inferiority clinical trial. Reg Anesth Pain Med. 2022;47:527–33. - PubMed
-
- Zhang H, Qu Z, Miao Y, Zhang Y, Qian L, Hua B, et al. Comparison between ultrasound-guided multi-injection intertransverse process and thoracic paravertebral blocks for major breast cancer surgery: a randomized non-inferiority trial. Reg Anesth Pain Med. 2023;48:161–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

