A viral vaccine design harnessing prior BCG immunization confers protection against Ebola virus
- PMID: 39081315
- PMCID: PMC11286471
- DOI: 10.3389/fimmu.2024.1429909
A viral vaccine design harnessing prior BCG immunization confers protection against Ebola virus
Abstract
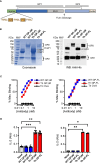
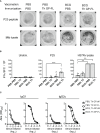
Previous studies have demonstrated the efficacy and feasibility of an anti-viral vaccine strategy that takes advantage of pre-existing CD4+ helper T (Th) cells induced by Mycobacterium bovis bacille Calmette-Guérin (BCG) vaccination. This strategy uses immunization with recombinant fusion proteins comprised of a cell surface expressed viral antigen, such as a viral envelope glycoprotein, engineered to contain well-defined BCG Th cell epitopes, thus rapidly recruiting Th cells induced by prior BCG vaccination to provide intrastructural help to virus-specific B cells. In the current study, we show that Th cells induced by BCG were localized predominantly outside of germinal centers and promoted antibody class switching to isotypes characterized by strong Fc receptor interactions and effector functions. Furthermore, BCG vaccination also upregulated FcγR expression to potentially maximize antibody-dependent effector activities. Using a mouse model of Ebola virus (EBOV) infection, this vaccine strategy provided sustained antibody levels with strong IgG2c bias and protection against lethal challenge. This general approach can be easily adapted to other viruses, and may be a rapid and effective method of immunization against emerging pandemics in populations that routinely receive BCG vaccination.
Keywords: CD4+ T cells; Ebola virus; Fc receptors; Mycobacterium bovis BCG; antibodies; intrastructural help; vaccines.
Copyright © 2024 Ng, Furuyama, Wirchnianski, Saavedra-Ávila, Johndrow, Chandran, Jacobs, Marzi and Porcelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Update of
-
A viral vaccine design harnessing prior BCG immunization confers protection against Ebola virus.bioRxiv [Preprint]. 2024 May 29:2024.05.28.595735. doi: 10.1101/2024.05.28.595735. bioRxiv. 2024. Update in: Front Immunol. 2024 Jul 16;15:1429909. doi: 10.3389/fimmu.2024.1429909. PMID: 38853867 Free PMC article. Updated. Preprint.
References
-
- Mohammed I, Nauman A, Paul P, Ganesan S, Chen KH, Jalil SMS, et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother. (2022) 18:2027160. doi: 10.1080/21645515.2022.2027160 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

