Unveiling the potential of novel indol-3-yl-phenyl allylidene hydrazine carboximidamide derivatives as AChE/BACE 1 dual inhibitors: a combined in silico, synthesis and in vitro study
- PMID: 39081657
- PMCID: PMC11287240
- DOI: 10.1039/d4ra04315d
Unveiling the potential of novel indol-3-yl-phenyl allylidene hydrazine carboximidamide derivatives as AChE/BACE 1 dual inhibitors: a combined in silico, synthesis and in vitro study
Abstract
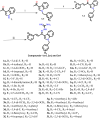
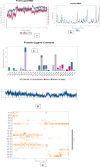
Considering the failure of many enzyme inhibitors for Alzheimer's disease (AD), research is now focused on multi-target directed drug discovery. In this paper, inhibition of two essential enzymes implicated in AD pathologies, acetylcholinesterase (AChE) and BACE 1 (Beta-site APP Cleaving Enzyme), has been explored. Taking clues from our previous work, 41 novel indol-3-yl phenyl allylidene hydrazine carboximidamide derivatives were synthesized. The results indicated that compounds inhibited both enzymes in micromolar concentrations. Compound 1l is proposed as the most active. In silico, it was seen to occupy the binding pocket of AChE and BACE 1. The ADME predictions showed that these compounds have acceptable physicochemical characteristics. This study provides new leads for the assessment of AChE and BACE 1 dual inhibition as a promising strategy for AD treatment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Sang Z. Wang K. Dong J. Tang L. Alzheimer's Disease: Updated Multi-targets Therapeutics Are in Clinical and in Progress. Eur. J. Med. Chem. 2022;238:114464. - PubMed
-
- https://www.who.int, accessed on 04-04-2024
-
- Luo Z. Sheng J. Sun Y. Lu C. Yan J. Liu A. Luo H.-b. Huang L. Li X. Synthesis and evaluation of multi-target-directed ligands against Alzheimer's disease based on the fusion of donepezil and ebselen. J. Med. Chem. 2013;56:9089–9099. - PubMed
-
- Srivastava P. Tripathi P. N. Sharma P. Shrivastava S. K. Design, Synthesis, and Evaluation of Novel N-(4-phenoxybenzyl)Aniline Derivatives Targeting Acetylcholinesterase, β-amyloid Aggregation and Oxidative Stress to Treat Alzheimer’S Disease. Bioorg. Med. Chem. 2019;27:3650–3662. - PubMed
LinkOut - more resources
Full Text Sources

