CXCL6-CXCR2 axis-mediated PD-L2+ mast cell accumulation shapes the immunosuppressive microenvironment in osteosarcoma
- PMID: 39082021
- PMCID: PMC11284376
- DOI: 10.1016/j.heliyon.2024.e34290
CXCL6-CXCR2 axis-mediated PD-L2+ mast cell accumulation shapes the immunosuppressive microenvironment in osteosarcoma
Abstract
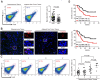
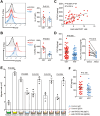
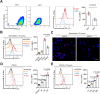
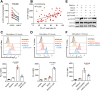
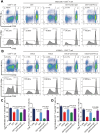
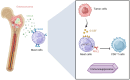
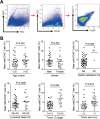
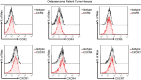
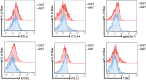
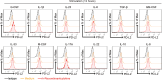
Osteosarcoma (OS) is the most common primary bone malignancy and has a high propensity for local invasion and metastasis. The tumour microenvironment of OS is infiltrated by a large number of immune cells, which play a crucial role in its progression and prognosis. Mast cells are important innate immune cells in the tumour stroma and exhibit different phenotypes in diverse tumour microenvironments. However, the underlying mechanisms of mast cell accumulation and the phenotypic characteristics of mast cells in OS remain poorly understood. In this article, we found for the first time that mast cell accumulation in osteosarcoma tissue was modulated by the CXCL6-CXCR2 axis and that the number of infiltrating mast cells was significantly greater in tumour tissues than in adjacent nontumour tissues. These tumour-infiltrating mast cells express high levels of the immunosuppressive molecule PD-L2, and survival analyses revealed that patients in the PD-L2+ high-expression group had a worse prognosis. In vitro, mast cells were induced to express PD-L2 in a time- and dose-dependent manner using OS tissue culture supernatants to mimic the tumour microenvironment. Mechanistic studies revealed that tumour cell-derived G-CSF significantly induced mast cell PD-L2 expression by activating STAT3. Importantly, mast cells overexpressing PD-L2 inhibit tumour-specific CD8+ T-cell proliferation and tumour-killing cytokine secretion, which is reversed by blocking PD-L2 on mast cells. Therefore, our findings provide new insight into the immunosuppressive and tumorigenic roles of mast cells, as well as a novel mechanism by which PD-L2-expressing mast cells mediate immune tolerance.
Keywords: Immunosuppressive; Mast cells; Osteosarcoma; PD-L2.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
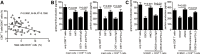

Similar articles
-
Clinical significance of programmed cell death-ligand expression in small bowel adenocarcinoma is determined by the tumor microenvironment.World J Gastroenterol. 2023 Oct 28;29(40):5566-5581. doi: 10.3748/wjg.v29.i40.5566. World J Gastroenterol. 2023. PMID: 37970475 Free PMC article.
-
Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-α-PD-L1 pathway.J Immunother Cancer. 2019 Feb 26;7(1):54. doi: 10.1186/s40425-019-0530-3. J Immunother Cancer. 2019. PMID: 30808413 Free PMC article.
-
FasL+ PD-L2+ Identifies a Novel Immunosuppressive Neutrophil Population in Human Gastric Cancer That Promotes Disease Progression.Adv Sci (Weinh). 2022 Feb;9(5):e2103543. doi: 10.1002/advs.202103543. Epub 2021 Dec 26. Adv Sci (Weinh). 2022. PMID: 34957697 Free PMC article.
-
Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer.Int J Mol Sci. 2019 Apr 29;20(9):2106. doi: 10.3390/ijms20092106. Int J Mol Sci. 2019. PMID: 31035644 Free PMC article. Review.
-
The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma.Cell Immunol. 2019 Sep;343:103711. doi: 10.1016/j.cellimm.2017.10.011. Epub 2017 Nov 2. Cell Immunol. 2019. PMID: 29117898 Review.
Cited by
-
The Crosstalk with CXCL10-Rich Tumor-Associated Mast Cells Fuels Pancreatic Cancer Progression and Immune Escape.Adv Sci (Weinh). 2025 Apr;12(14):e2417724. doi: 10.1002/advs.202417724. Epub 2025 Feb 18. Adv Sci (Weinh). 2025. PMID: 39965084 Free PMC article.
-
Prognostic and immunological characterization of osteosarcoma patients evaluated by liquid-liquid phase separation related genes.Discov Oncol. 2025 May 30;16(1):958. doi: 10.1007/s12672-025-02769-9. Discov Oncol. 2025. PMID: 40445434 Free PMC article.
-
Tumor-derived G-CSF induces an immunosuppressive microenvironment in an osteosarcoma model, reducing response to CAR.GD2 T-cells.J Hematol Oncol. 2024 Dec 18;17(1):127. doi: 10.1186/s13045-024-01641-7. J Hematol Oncol. 2024. PMID: 39695851 Free PMC article.
-
WDR3 undergoes phase separation to mediate the therapeutic mechanism of Nilotinib against osteosarcoma.J Exp Clin Cancer Res. 2025 Jul 11;44(1):201. doi: 10.1186/s13046-025-03456-x. J Exp Clin Cancer Res. 2025. PMID: 40646517 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

