A BN-Benzvalene
- PMID: 39082667
- PMCID: PMC12087574
- DOI: 10.1021/jacs.4c08088
A BN-Benzvalene
Abstract
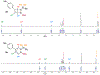
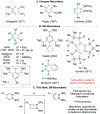
The synthesis and crystallographic characterization of BN-benzvalene, the first second-row heteroatom-containing benzvalene, is described. BN-benzvalenes are produced via photoexcitation of C5-aryl-substituted 1,2-azaborines under flow conditions. Mechanistic studies support a boron-specific, two-step photoisomerization pathway involving a BN-Dewar benzene intermediate, which is distinct from the photoisomerization pathway proposed in benzene and phospha- and silabenzenes for the formation of their respective benzvalene analogues.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures


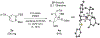


References
-
- For an overview, see:
- Bolesov IG Valence Isomers of Benzene. Russ. Chem. Rev 1968, 37, 666–670.
- Van Tamelen EE Valence-Bond Isomers of Aromatic Systems. Angew. Chem., Int. Ed 1965, 4, 738–745.
- Scott LT; Jones M Rearrangements and Interconversions of Compounds of the Formula (CH)n. Chem. Rev 1972, 72, 181–202.
-
- Wilzbach KE; Ritscher JS; Kaplan L Benzvalene, the Tricyclic Valence Isomer of Benzene. J. Am. Chem. Soc 1967, 89, 1031–1032.
- Christl M Benzvalene—Properties and Synthetic Potential. Angew. Chem., Int. Ed 1981, 20, 529–546.
-
- Dewar J 5. On the Oxidation of Phenyl Alcohol, and a Mechanical Arrangement adapted to illustrate Structure in the Nonsaturated Hydrocarbons. Proc. R. Soc. Edinburgh 1869, 6, 82–86.
- Van Tamelen EE; Pappas SP Chemistry of Dewar Benzene. 1,2,5-Tri-t-Butylbicyclo[2.2.0]Hexa-2,5-Diene. J. Am. Chem. Soc 1962, 84, 3789–3791.
- Van Tamelen EE; Pappas SP Bicyclo [2.2.0]hexa-2,5-diene. J. Am. Chem. Soc 1963, 85, 3297–3298.
- Van Tamelen EE; Pappas SP; Kirk KL Valence bond isomers of aromatic systems. Bicyclo[2.2.0]hexa-2,5-dienes (Dewar benzenes). J. Am. Chem. Soc 1971, 93, 6092–6101.
-
- Ladenburg A Bemerkungen zur aromatischen Theorie. Ber. Dtsch. Chem. Ges 1869, 2, 140–142.
- Katz TJ; Acton N Synthesis of prismane. J. Am. Chem. Soc 1973, 95, 2738–2739.
-
- Billups WE; Haley MM Bicycloprop-2-enyl (C6H6). Angew. Chem., Int. Ed 1989, 28, 1711–1712.
- Boese R; Bläser D; Gleiter R; Pfeifer KH; Billups WE; Haley MM Structure and photoelectron spectrum of 3,3′-bicyclopropenyl. J. Am. Chem. Soc 1993, 115, 743–746.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

