Maintenance with mirvetuximab soravtansine plus bevacizumab vs bevacizumab in FRα-high platinum-sensitive ovarian cancer
- PMID: 39082675
- PMCID: PMC11520569
- DOI: 10.1080/14796694.2024.2372241
Maintenance with mirvetuximab soravtansine plus bevacizumab vs bevacizumab in FRα-high platinum-sensitive ovarian cancer
Abstract
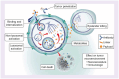
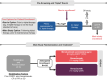
At first recurrence, platinum-sensitive ovarian cancer (PSOC) is frequently treated with platinum-based chemotherapy doublets plus bevacizumab, then single-agent bevacizumab. Most patients' disease progresses within a year after chemotherapy, emphasizing the need for novel strategies. Mirvetuximab soravtansine-gynx (MIRV), an antibody-drug conjugate, comprises a folate receptor alpha (FRα)-binding antibody and tubulin-targeting payload (maytansinoid DM4). In FRα-high PSOC, MIRV plus bevacizumab previously showed promising efficacy (objective response rate, 69% [95% CI: 41-89]; median progression-free survival, 13.3 months [95% CI: 8.3-18.3]; median duration of response, 12.9 months [95% CI: 6.5-15.7]) and safety. The Phase III randomized GLORIOSA trial will evaluate MIRV plus bevacizumab vs. bevacizumab alone as maintenance therapy in patients with FRα-high PSOC who did not have disease progression following second-line platinum-based doublet chemotherapy plus bevacizumab.Clinical Trial Registration: ClinicalTrials.gov ID: NCT05445778; GOG.org ID: GOG-3078; ENGOT.ESGO.org ID: ENGOT-ov76.
Keywords: GLORIOSA trial; bevacizumab; folate receptor alpha; maintenance therapy; mirvetuximab soravtansine; platinum-sensitive ovarian cancer.
Plain language summary
Most patients with ovarian cancer are initially treated with platinum-based chemotherapy. If the cancer reappears/recurs after more than 6 months following this therapy, it is called platinum-sensitive ovarian cancer (PSOC). Patients with PSOC usually receive additional platinum-based chemotherapy along with bevacizumab, a drug that reduces tumor growth by decreasing its blood supply. If patients improve or are stable on this therapy, they are usually kept on bevacizumab alone for ‘maintenance therapy’. Unfortunately, this maintenance therapy does not work long-term in all patients, so better long-term treatments are needed. The GLORIOSA (NCT05445778) clinical trial will compare maintenance therapy with bevacizumab alone to maintenance therapy with bevacizumab plus a drug called mirvetuximab soravtansine-gynx (MIRV) to determine which therapy leads to better results in patients with PSOC. MIRV is made up of an antibody that binds to a specific protein (folate receptor alpha [FRα]) on cancer cells to directly deliver a cancer-killing drug. MIRV received US FDA approval to be used as a therapy for patients with ovarian cancer who are resistant to platinum-based chemotherapy and express high levels of FRα. The GLORIOSA trial will study maintenance therapy with MIRV plus bevacizumab in patients with PSOC who have not had cancer progression after second-line platinum-based chemotherapy plus bevacizumab, and whose cancer expresses high amounts of FRα. The main purpose of this trial is to determine if MIRV plus bevacizumab leads to better patient survival and decreases cancer growth and spread when compared with bevacizumab alone.
Conflict of interest statement
DM O'Malley: Funding for clinical research from AbbVie Inc, Agenus Inc, Ajinomoto Co, Inc, Amgen Inc, Array BioPharma Inc, AstraZeneca Pharmaceuticals LP, Bristol Myers Squibb Company, Clovis Oncology, Inc, Daré Bioscience, Inc, Eisai Co, Ltd, EMD Serono, Ergomed plc, Genentech, Inc, Genmab A/S, The GOG Foundation Inc, ImmunoGen, Inc, Iovance Biotherapeutics, Inc, Janssen Biotech, Inc, Johnson & Johnson Pharmaceuticals, Ludwig Institute for Cancer Research Ltd, Merck & Co, Inc, Merck Serono, Mersana Therapeutics, Inc, New Mexico Cancer Care Alliance, Novocure GmbH, PRA Health Sciences, Regeneron Pharmaceuticals, Inc, Seagen Inc, Stemcentrx, Inc, Sumitomo Dainippon Pharma Oncology, Inc, Syneos Health, TESARO Inc, TRACON Pharmaceuticals, Inc, VentiRx Pharmaceuticals, Inc and Yale University; personal fees from Agenus Inc, Myriad Genetics, Inc, Rubius Therapeutics, Inc and Tarveda Therapeutics; and fees for consulting or advisory boards from AbbVie Inc, Ambry Genetics Corporation, Amgen Inc, Arquer Diagnostics Ltd, AstraZeneca Pharmaceuticals LP, Celsion Corporation, Clovis Oncology, Inc, Corcept Therapeutics Incorporated, Eisai Co, Ltd, Elevar Therapeutics, Inc, Genentech, Inc, The GOG Foundation, Inc, ImmunoGen, Inc, InxMed Co, Ltd, Iovance Biotherapeutics, Inc, Janssen Biotech, Inc, Johnson & Johnson Pharmaceuticals, Merck & Co, Inc, Mersana Therapeutics, Inc, Novartis, Novocure GmbH, Regeneron Pharmaceuticals, Inc, Roche Diagnostics MSA, Seagen Inc, Sorrento Therapeutics, Inc, Sumitomo Dainippon Pharma Oncology, Inc, Takeda Pharmaceuticals USA, Inc, TESARO Inc and Toray Industries, Inc.
T Myers: Honoraria for lecture to ImmunoGen, Inc. Institutional research support from GSK.
P Wimberger: Research funding from Amgen Inc, AstraZeneca, MSD, GlaxoSmithKline, Novartis, Pfizer Inc, Roche Pharma, Clovis Oncology, Inc and Eli Lilly and Company and honoraria from Amgen Inc, AstraZeneca, MSD, GlaxoSmithKline, Novartis, Pfizer Inc, Roche Pharma, Clovis Oncology, Inc, Teva Pharmaceutical Industries Ltd, Eisai Co, Ltd, Eli Lilly and Company, Gilead Sciences, Inc and Daiichi Sankyo Company, Limited. Participation in advisory boards for Amgen Inc, AstraZeneca, MSD, GlaxoSmithKline, Novartis, Pfizer Inc, Roche Pharma, Clovis Oncology, Inc, Teva Pharmaceutical Industries Ltd, Eisai Co, Ltd, Eli Lilly and Company, Gilead Sciences, Inc and Daiichi Sankyo Company, Limited.
T Van Gorp: Consulting/advising with AstraZeneca, BioNTech SE, Eisai Co, Ltd, GSK, ImmunoGen, Inc, Incyte Corporation, MSD/Merck & Co, Inc, OncXerna Therapeutics, Inc, Seagen Inc, Tubulis GmbH and Zentalis Pharmaceuticals, Inc. Travel, accommodations, and/or expenses from AstraZeneca, GSK, ImmunoGen, Inc, MSD/Merck and PharmaMar. Research funding from Amgen Inc, AstraZeneca and Roche. All payments institutional.
A Redondo: Honoraria, advisory/consultancy services and speakers bureau for MSD, AstraZeneca, GSK and PharmaMar. Travel/accommodations/expenses from AstraZeneca, GSK and PharmaMar.
D Cibula: Advisory board participation with Akeso Bio, AstraZeneca, GSK, MSD, Novocure GmbH, Roche, Seagen Inc and Sotio Biotech. Travel expenses from AstraZeneca.
S Nicum: Institutional research funding from AstraZeneca and GSK. Participation in advisory boards, as a speaker, and/or on a steering committee for AstraZeneca, Clovis Oncology, Inc and GSK.
M Rodrigues: Honoraria: Immunocore Ltd; consulting or advisory role: AstraZeneca and GlaxoSmithKline; research funding: Daiichi Sankyo Company, Limited/AstraZeneca; and travel, accommodations and expenses: AstraZeneca.
FJ Backes: Grant/research/clinical trial support: Clovis Oncology, Inc; Eisai Co, Ltd; ImmunoGen, Inc; Merck; and Natera, Inc. Consultant/advisory boards: Agenus Inc; AstraZeneca; Clovis Oncology, Inc; Eisai Co, Ltd; GlaxoSmithKline; ImmunoGen, Inc; and Merck.
JN Barlin: Consulting or advisory role: AstraZeneca, Clovis Oncology, Inc and OncoC4. Speakers bureau: AstraZeneca, Clovis Oncology, Inc and Merck.
SN Lewin: Research funding from Tesoro, The GOG Foundation Inc, Merck and ImmunoGen, Inc. Speakers bureau for ImmunoGen, Inc, AstraZeneca, GSK, Merck and Myriad Genetic Laboratories Inc.
P Lim: Nothing to disclose.
B Pothuri: Grants, consulting and advisory board fees; institutional PI for industry-sponsored trials from Tesaro Inc/GSK, Duality Biologics, AstraZeneca, Merck, Genentech, Inc/Roche, Celsion Corporation, ImmunoGen, Inc, Karyopharm Therapeutics Inc, Mersana Therapeutics, Inc, Takeda Pharmaceutical Company Limited, Toray, I-Mab Biopharma Co, Ltd, Sutro Biopharma, Inc, Seagen Inc and Clovis Oncology, Inc. Compensated advisory boards include Tesaro Inc/GSK, AstraZeneca, Eli Lilly and Company, Mersana Therapeutics, Inc, Onconova Therapeutics, Inc, Merck, Clovis Oncology, Inc, Eisai Co, Ltd, Toray, Sutro Biopharma, Inc, Deciphera Pharmaceuticals, Inc, I-Mab Biopharma Co, Ltd, Seagen Inc, Imvax, Inc and The GOG Foundation Inc.
E Diver: Employment by ImmunoGen, Inc.
S Banerjee: Institutional research funding from AstraZeneca and GlaxoSmithKline; consulting fees from AstraZeneca, Epsilogen Ltd, GlaxoSmithKline, ImmunoGen, Inc, Merck Sharp & Dohme, Mersana Therapeutics, Inc, Novartis, OncXerna Therapeutics, Inc, Seagen Inc, Shattuck Labs, Inc and Regeneron Pharmaceuticals, Inc; and honoraria from Amgen Inc, AstraZeneca, Clovis Oncology, Inc, GlaxoSmithKline, ImmunoGen, Inc, Merck Sharp & Dohme, Mersana Therapeutics, Inc, Novocure GmbH, Pfizer Inc, Roche and Takeda Pharmaceutical Company Limited.
D Lorusso: Research funding and consulting or advisory fees: PharmaMar, Clovis Oncology, Inc, GSK, MSD, AstraZeneca, Amgen Inc, Seagen Inc/Genmab A/S, ImmunoGen, Inc and Merck Serono. Research funding: Incyte Corporation, Novartis, Roche, Sutro Biopharma, Inc and Corcept Therapeutics Inc.
The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

References
-
- Moore KN, Martin LP, O'Malley DM, et al. . A review of mirvetuximab soravtansine in the treatment of platinum-resistant ovarian cancer. Future Oncol. 2018;14(2):123–136. doi:10.2217/fon-2017-0379 - DOI - PubMed
-
• Review of mechanism of action, pharmacology and clinical evaluation of mirvetuximab soravtansine in ovarian cancer.
-
- Moore KN, Martin LP, O'Malley DM, et al. . Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor α-targeting antibody–drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a Phase I expansion study. J Clin Oncol. 2017;35(10):1112–1118. doi:10.1200/JCO.2016.69.9538 - DOI - PMC - PubMed
-
- World Health Organization, International Agency for Research on Cancer . Ovary. https://gco.iarc.fr/today/data/factsheets/cancers/25-Ovary-fact-sheet.pdf (Accessed December 1, 2023).
-
- Globocan 2020 Europe . International Agency for Research on Cancer. Lyon, France: World Health Organization; 2020. Fact Sheet 908.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
