A Phase 3 Clinical Study to Evaluate the Safety, Tolerability, and Immunogenicity of V116 in Pneumococcal Vaccine-Experienced Adults 50 Years of Age or Older (STRIDE-6)
- PMID: 39082735
- PMCID: PMC11650886
- DOI: 10.1093/cid/ciae383
A Phase 3 Clinical Study to Evaluate the Safety, Tolerability, and Immunogenicity of V116 in Pneumococcal Vaccine-Experienced Adults 50 Years of Age or Older (STRIDE-6)
Abstract
Background: Pneumococcal diseases cause considerable morbidity and mortality in adults. V116 is an investigational 21-valent pneumococcal conjugate vaccine (PCV) specifically designed to protect adults from pneumococcal serotypes responsible for the majority of residual pneumococcal diseases. This phase 3 study evaluated safety, tolerability, and immunogenicity of V116 in pneumococcal vaccine-experienced adults aged ≥50 years.
Methods: A total of 717 adults were enrolled to receive a single dose of pneumococcal vaccine as follows: cohort 1 (n = 350) previously received 23-valent pneumococcal polysaccharide vaccine (PPSV23) and were randomized 2:1 to receive V116 or PCV15, respectively; cohort 2 (n = 261) previously received PCV13 and were randomized 2:1 to receive V116 or PPSV23, respectively; cohort 3 (n = 106) previously received PPSV23 + PCV13, PCV13 + PPSV23, PCV15 + PPSV23, or PCV15 and all received open-label V116. Immunogenicity was evaluated 30 days postvaccination using opsonophagocytic activity (OPA) geometric mean titers (GMTs) and immunoglobulin G (IgG) geometric mean concentrations (GMCs) for all V116 serotypes. Safety was evaluated as the proportion of participants with adverse events (AEs).
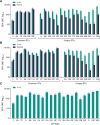
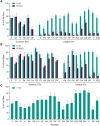
Results: V116 was immunogenic across all 3 cohorts as assessed by serotype-specific OPA GMTs and IgG GMCs postvaccination for all 21 serotypes. V116 elicited comparable immune responses to serotypes shared with PCV15 (cohort 1) or PPSV23 (cohort 2), and higher immune responses to serotypes unique to V116. The proportions of participants with solicited AEs were generally comparable across cohorts.
Conclusions: V116 is well tolerated with a safety profile comparable to currently licensed pneumococcal vaccines and generates IgG and functional immune responses to all V116 serotypes, regardless of prior pneumococcal vaccine received.
Clinical trials registration: NCT05420961; EudraCT 2021-006679-41.
Keywords: V116; adult; pneumococcal; safety; vaccine.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. P. S., T. Z., J. L., H. L. P., D. F., and N. G. are employees of MSD and may hold stock in Merck & Co., Inc., Rahway, New Jersey, USA. M. D. holds stock in MSD. C. G. G. has received grants or contracts from the Centers for Disease Control and Prevention, Agency for Healthcare Research and Quality, Food and Drug Administration, Syneos Health, and National Institutes of Health. W. A. O. has received grants or contracts from Sanofi, Seqirus, and Moderna. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- LeBlanc JJ, ElSherif M, Ye L, et al. Burden of vaccine-preventable pneumococcal disease in hospitalized adults: a Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS) network study. Vaccine 2017; 35:3647–54. - PubMed
-
- Pick H, Daniel P, Rodrigo C, et al. Pneumococcal serotype trends, surveillance and risk factors in UK adult pneumonia, 2013–18. Thorax 2020; 75:38–49. - PubMed
-
- Simonetti AF, van Werkhoven CH, Schweitzer VA, et al. Predictors for individual patient antibiotic treatment effect in hospitalized community-acquired pneumonia patients. Clin Microbiol Infect 2017; 23:774.e1–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

