Host range of naturally and artificially evolved symbiotic bacteria for a specific host insect
- PMID: 39082826
- PMCID: PMC11389372
- DOI: 10.1128/mbio.01342-24
Host range of naturally and artificially evolved symbiotic bacteria for a specific host insect
Abstract
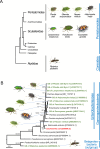
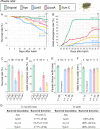
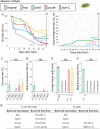
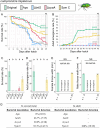
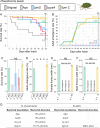
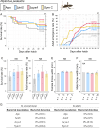
Diverse insects are intimately associated with specific symbiotic bacteria, where host and symbiont are integrated into an almost inseparable biological entity. These symbiotic bacteria usually exhibit host specificity, uncultivability, reduced genome size, and other peculiar traits relevant to their symbiotic lifestyle. How host-symbiont specificity is established at the very beginning of symbiosis is of interest but poorly understood. To gain insight into the evolutionary issue, we adopted an experimental approach using the recently developed evolutionary model of symbiosis between the stinkbug Plautia stali and Escherichia coli. Based on the laboratory evolution of P. stali-E. coli mutualism, we selected ΔcyaA mutant of E. coli as an artificial symbiont of P. stali that has established mutualism by a single mutation. In addition, we selected a natural cultivable symbiont of P. stali of relatively recent evolutionary origin. These artificial and natural symbiotic bacteria of P. stali were experimentally inoculated to symbiont-deprived newborn nymphs of diverse stinkbug species. Strikingly, the mutualistic E. coli was unable to establish infection and support growth and survival of all the stinkbug species except for P. stali, uncovering that host specificity can be established at a very early stage of symbiotic evolution. Meanwhile, the natural symbiont was able to establish infection and support growth and survival of several stinkbug species in addition to P. stali, unveiling that a broader host range of the symbiont has evolved in nature. Based on these findings, we discuss what factors are relevant to the establishment of host specificity in the evolution of symbiosis.IMPORTANCEHow does host-symbiont specificity emerge at the very beginning of symbiosis? This question is difficult to address because it is generally difficult to directly observe the onset of symbiosis. However, recent development of experimental evolutionary approaches to symbiosis has brought about a breakthrough. Here we tackled this evolutionary issue using a symbiotic Escherichia coli created in laboratory and a natural Pantoea symbiont, which are both mutualistic to the stinkbug Plautia stali. We experimentally replaced essential symbiotic bacteria of diverse stinkbugs with the artificial and natural symbionts of P. stali and evaluated whether the symbiotic bacteria, which evolved for a specific host, can establish infection and support the growth and survival of heterospecific hosts. Strikingly, the artificial symbiont showed strict host specificity to P. stali, whereas the natural symbiont was capable of symbiosis with diverse stinkbugs, which provide insight into how host-symbiont specificity can be established at early evolutionary stages of symbiosis.
Keywords: Escherichia coli; Pantoea; Plautia stali; gut symbiosis; host specificity; stinkbug.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Mechanisms Underpinning Morphogenesis of a Symbiotic Organ Specialized for Hosting an Indispensable Microbial Symbiont in Stinkbugs.mBio. 2023 Apr 25;14(2):e0052223. doi: 10.1128/mbio.00522-23. Epub 2023 Apr 5. mBio. 2023. PMID: 37017516 Free PMC article.
-
Peculiar structural features of midgut symbiotic organ in the early development of the stinkbug Plautia stali Scott, 1874 (Hemiptera: Pentatomidae).Naturwissenschaften. 2025 Apr 29;112(3):34. doi: 10.1007/s00114-025-01986-0. Naturwissenschaften. 2025. PMID: 40299062
-
Regulation and remodeling of microbial symbiosis in insect metamorphosis.Proc Natl Acad Sci U S A. 2023 Oct 3;120(40):e2304879120. doi: 10.1073/pnas.2304879120. Epub 2023 Sep 28. Proc Natl Acad Sci U S A. 2023. PMID: 37769258 Free PMC article.
-
Symbiotic factors in Burkholderia essential for establishing an association with the bean bug, Riptortus pedestris.Arch Insect Biochem Physiol. 2015 Jan;88(1):4-17. doi: 10.1002/arch.21218. Arch Insect Biochem Physiol. 2015. PMID: 25521625 Review.
-
Riptortus pedestris and Burkholderia symbiont: an ideal model system for insect-microbe symbiotic associations.Res Microbiol. 2017 Apr;168(3):175-187. doi: 10.1016/j.resmic.2016.11.005. Epub 2016 Dec 10. Res Microbiol. 2017. PMID: 27965151 Review.
References
-
- Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York.
-
- Bourtzis K, Miller TA. 2003. Insect symbiosis. CRC Press, Boca Raton, FL.
-
- Douglas AE. 2022. Insects and their beneficial microbes. Princeton University Press, Princeton, NJ.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
