Dissecting structure and function of the monovalent cation/H+ antiporters Mdm38 and Ylh47 in Saccharomyces cerevisiae
- PMID: 39082862
- PMCID: PMC11340316
- DOI: 10.1128/jb.00182-24
Dissecting structure and function of the monovalent cation/H+ antiporters Mdm38 and Ylh47 in Saccharomyces cerevisiae
Abstract
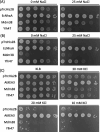
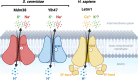
Saccharomyces cerevisiae Mdm38 and Ylh47 are homologs of the Ca2+/H+ antiporter Letm1, a candidate gene for seizures associated with Wolf-Hirschhorn syndrome in humans. Mdm38 is important for K+/H+ exchange across the inner mitochondrial membrane and contributes to membrane potential formation and mitochondrial protein translation. Ylh47 also localizes to the inner mitochondrial membrane. However, knowledge of the structures and detailed transport activities of Mdm38 and Ylh47 is limited. In this study, we conducted characterization of the ion transport activities and related structural properties of Mdm38 and Ylh47. Growth tests using Na+/H+ antiporter-deficient Escherichia coli strain TO114 showed that Mdm38 and Ylh47 had Na+ efflux activity. Measurement of transport activity across E. coli-inverted membranes showed that Mdm38 and Ylh47 had K+/H+, Na+/H+, and Li+/H+ antiport activity, but unlike Letm1, they lacked Ca2+/H+ antiport activity. Deletion of the ribosome-binding domain resulted in decreased Na+ efflux activity in Mdm38. Structural models of Mdm38 and Ylh47 identified a highly conserved glutamic acid in the pore-forming membrane-spanning region. Replacement of this glutamic acid with alanine, a non-polar amino acid, significantly impaired the ability of Mdm38 and Ylh47 to complement the salt sensitivity of E. coli TO114. These findings not only provide important insights into the structure and function of the Letm1-Mdm38-Ylh47 antiporter family but by revealing their distinctive properties also shed light on the physiological roles of these transporters in yeast and animals.
Importance: The inner membrane of mitochondria contains numerous ion transporters, including those facilitating H+ transport by the electron transport chain and ATP synthase to maintain membrane potential. Letm1 in the inner membrane of mitochondria in animals functions as a Ca2+/H+ antiporter. However, this study reveals that homologous antiporters in mitochondria of yeast, Mdm38 and Ylh47, do not transport Ca2+ but instead are selective for K+ and Na+. Additionally, the identification of conserved amino acids crucial for antiporter activity further expanded our understanding of the structure and function of the Letm1-Mdm38-Ylh47 antiporter family.
Keywords: S. cerevisiae; cation/H+ antiporter; mitochondria; yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Mdm38 interacts with ribosomes and is a component of the mitochondrial protein export machinery.J Cell Biol. 2006 Feb 13;172(4):553-64. doi: 10.1083/jcb.200505060. J Cell Biol. 2006. PMID: 16476776 Free PMC article.
-
Mdm38 is a 14-3-3-like receptor and associates with the protein synthesis machinery at the inner mitochondrial membrane.Traffic. 2011 Oct;12(10):1457-66. doi: 10.1111/j.1600-0854.2011.01239.x. Epub 2011 Jul 22. Traffic. 2011. PMID: 21718401
-
Functional expression in Escherichia coli of low-affinity and high-affinity Na(+)(Li(+))/H(+) antiporters of Synechocystis.J Bacteriol. 2001 Feb;183(4):1376-84. doi: 10.1128/JB.183.4.1376-1384.2001. J Bacteriol. 2001. PMID: 11157951 Free PMC article.
-
Monovalent cation transporters at the plasma membrane in yeasts.Yeast. 2019 Apr;36(4):177-193. doi: 10.1002/yea.3355. Epub 2018 Oct 3. Yeast. 2019. PMID: 30193006 Review.
-
Structure and function of yeast and fungal Na+ /H+ antiporters.IUBMB Life. 2018 Jan;70(1):23-31. doi: 10.1002/iub.1701. Epub 2017 Dec 8. IUBMB Life. 2018. PMID: 29219228 Review.
References
-
- Maathuis F, Sanders D. 1993. Energization of potassium uptake in Arabidopsis thaliana. Planta 191:302–307. doi:10.1007/BF00195686 - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 20KK0127/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21H05266/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21KK0268/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22K19121/MEXT | Japan Society for the Promotion of Science (JSPS)
- 23K13862/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Miscellaneous

