Amphibian mast cells serve as barriers to chytrid fungus infections
- PMID: 39082933
- PMCID: PMC11290838
- DOI: 10.7554/eLife.92168
Amphibian mast cells serve as barriers to chytrid fungus infections
Abstract
Global amphibian declines are compounded by deadly disease outbreaks caused by the chytrid fungus, Batrachochytrium dendrobatidis (Bd). Much has been learned about the roles of amphibian skin-produced antimicrobial components and microbiomes in controlling Bd, yet almost nothing is known about the roles of skin-resident immune cells in anti-Bd defenses. Mammalian mast cells reside within and serve as key immune sentinels in barrier tissues like skin. Accordingly, we investigated the roles of Xenopus laevis frog mast cells during Bd infections. Our findings indicate that enrichment of X. laevis skin mast cells confers anti-Bd protection and ameliorates the inflammation-associated skin damage caused by Bd infection. This includes a significant reduction in infiltration of Bd-infected skin by neutrophils, promoting mucin content within cutaneous mucus glands, and preventing Bd-mediated changes to skin microbiomes. Mammalian mast cells are known for their production of the pleiotropic interleukin-4 (IL4) cytokine and our findings suggest that the X. laevis IL4 plays a key role in manifesting the effects seen following cutaneous mast cell enrichment. Together, this work underscores the importance of amphibian skin-resident immune cells in anti-Bd defenses and illuminates a novel avenue for investigating amphibian host-chytrid pathogen interactions.
Keywords: amphibian immunity; chytrid fungus; immunology; inflammation; mast cells; skin immunity; xenopus.
© 2023, Hauser et al.
Conflict of interest statement
KH, CG, RC, MH, DH, NR, LG, AY, NK, MZ, EJ, AD, LR, CM, LG No competing interests declared
Figures



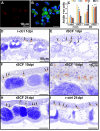

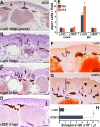
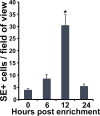


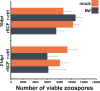


Update of
- doi: 10.1101/2023.09.29.560127
- doi: 10.7554/eLife.92168.1
- doi: 10.7554/eLife.92168.2
References
-
- Agis H, Beil WJ, Bankl HC, Füreder W, Sperr WR, Ghannadan M, Baghestanian M, Sillaber C, Bettelheim P, Lechner K, Valent P. Mast cell-lineage versus basophil lineage involvement in myeloproliferative and myelodysplastic syndromes: Diagnostic role of cell-immunopheno typing. Leukemia & Lymphoma. 1996;22:187–204. doi: 10.3109/10428199609051750. - DOI - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

