Noninferiority of 16-Week vs 8-Week Guselkumab Dosing in Super Responders for Maintaining Control of Psoriasis: The GUIDE Randomized Clinical Trial
- PMID: 39083288
- PMCID: PMC11292573
- DOI: 10.1001/jamadermatol.2024.2463
Noninferiority of 16-Week vs 8-Week Guselkumab Dosing in Super Responders for Maintaining Control of Psoriasis: The GUIDE Randomized Clinical Trial
Erratum in
-
Errors in Figures 1 and 3.JAMA Dermatol. 2024 Nov 1;160(11):1257. doi: 10.1001/jamadermatol.2024.4370. JAMA Dermatol. 2024. PMID: 39382861 Free PMC article. No abstract available.
Abstract
Importance: Psoriasis is a chronic inflammatory skin disease with unmet needs for tailored treatment and therapy de-escalation strategies.
Objective: To evaluate early intervention with and prolonging the dosing interval for guselkumab, a p19 subunit-targeted interleukin (IL)-23 inhibitor, in patients with moderate to severe psoriasis.
Design, setting, and participants: The GUIDE clinical trial is an ongoing phase 3b, randomized, double-blinded trial conducted across 80 centers in Germany and France comprising 3 parts evaluating the impact of early disease intervention, prolonged dosing interval, and maintenance of response following treatment withdrawal among adults with moderate to severe plaque psoriasis. In study part 2, reported herein, first and last patient visits were September 2019 and March 2022, respectively.
Interventions: In GUIDE part 1 (week [W]0-W28), patients received guselkumab, 100 mg, at W0, W4, W12, and W20. Those achieving a Psoriasis Area and Severity Index (PASI) of 0 at both W20 and W28 were termed super responders (SRes). In part 2 (W28-W68), SRes were randomized to guselkumab, 100 mg, every 8 weeks or every 16 weeks; non-SRes continued open-label guselkumab every 8 weeks.
Main outcomes and measures: Primary objective was to demonstrate noninferiority (with a 10% margin) of guselkumab every 16 weeks vs every 8 weeks dosing among SRes for maintenance of disease control (PASI <3 at W68). Biomarker substudies assessed immunologic effects in skin and blood.
Results: Overall, 822 patients received guselkumab in part 2 (297 [36.1%] SRes [every 8 weeks/every 16 weeks; n = 148/n = 149] and 525 [63.9%] non-SRes). Among SRes, mean (SD) age was 39.4 (14.1) years, 95 (32.0%) were female, and 202 (68.0%) were male. The primary end point of noninferiority for guselkumab every 16 weeks vs every 8 weeks in SRes was met (P = .001), with 91.9% (137/149; 90% CI, 87.3%-95.3%) of SRes receiving every 16 weeks and 92.6% (137/148; 90% CI, 88.0%-95.8%) of SRes receiving dosing every 8 weeks having PASI lower than 3 at W68. Clinical effects corresponded with immunologic changes; skin CD8-positive tissue-resident memory T (TRM)-cell count decreased quickly from baseline, remaining low in both dosing groups. Similarly, serum IL-17A, IL-17F, IL-22, and β defensin (BD)-2 levels decreased significantly from baseline, remaining low in both dosing groups to W68. Guselkumab was well-tolerated; no new safety signals were identified.
Conclusions and relevance: Psoriasis treatment guidelines lack or provide inconsistent advice on patient stratification and treatment de-escalation. We present the first randomized trial providing evidence that, in patients with early complete skin clearance at 2 consecutive visits (W20 and W28), extending the guselkumab dosing interval may control disease activity.
Trial registration: ClinicalTrials.gov Identifier: NCT03818035.
Conflict of interest statement
Figures


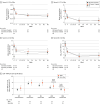
Comment on
-
Extending Maintenance Dosing Intervals for Guselkumab in the Treatment of Patients With Psoriasis.JAMA Dermatol. 2024 Sep 1;160(9):919-920. doi: 10.1001/jamadermatol.2024.2462. JAMA Dermatol. 2024. PMID: 39083259 No abstract available.
References
-
- Eyerich K, Weisenseel P, Pinter A, et al. IL-23 blockade with guselkumab potentially modifies psoriasis pathogenesis: rationale and study protocol of a phase 3b, randomised, double-blind, multicentre study in participants with moderate-to-severe plaque-type psoriasis (GUIDE). BMJ Open. 2021;11(9):e049822. doi: 10.1136/bmjopen-2021-049822 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

