Targeting the muscarinic M1 receptor with a selective, brain-penetrant antagonist to promote remyelination in multiple sclerosis
- PMID: 39083422
- PMCID: PMC11317586
- DOI: 10.1073/pnas.2407974121
Targeting the muscarinic M1 receptor with a selective, brain-penetrant antagonist to promote remyelination in multiple sclerosis
Erratum in
-
Correction for Poon et al., Targeting the muscarinic M1 receptor with a selective, brain-penetrant antagonist to promote remyelination in multiple sclerosis.Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2420080121. doi: 10.1073/pnas.2420080121. Epub 2024 Oct 21. Proc Natl Acad Sci U S A. 2024. PMID: 39432800 Free PMC article. No abstract available.
Expression of concern in
-
Editorial Expression of Concern for Poon et al., Targeting the muscarinic M1 receptor with a selective, brain-penetrant antagonist to promote remyelination in multiple sclerosis.Proc Natl Acad Sci U S A. 2025 Jun 17;122(24):e2512750122. doi: 10.1073/pnas.2512750122. Epub 2025 Jun 13. Proc Natl Acad Sci U S A. 2025. PMID: 40512803 Free PMC article. No abstract available.
Abstract
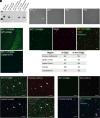
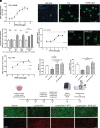
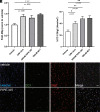
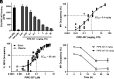
Multiple sclerosis (MS) is a chronic and debilitating neurological disease that results in inflammatory demyelination. While endogenous remyelination helps to recover function, this restorative process tends to become less efficient over time. Currently, intense efforts aimed at the mechanisms that promote remyelination are being considered promising therapeutic approaches. The M1 muscarinic acetylcholine receptor (M1R) was previously identified as a negative regulator of oligodendrocyte differentiation and myelination. Here, we validate M1R as a target for remyelination by characterizing expression in human and rodent oligodendroglial cells (including those in human MS tissue) using a highly selective M1R probe. As a breakthrough to conventional methodology, we conjugated a fluorophore to a highly M1R selective peptide (MT7) which targets the M1R in the subnanomolar range. This allows for exceptional detection of M1R protein expression in the human CNS. More importantly, we introduce PIPE-307, a brain-penetrant, small-molecule antagonist with favorable drug-like properties that selectively targets M1R. We evaluate PIPE-307 in a series of in vitro and in vivo studies to characterize potency and selectivity for M1R over M2-5R and confirm the sufficiency of blocking this receptor to promote differentiation and remyelination. Further, PIPE-307 displays significant efficacy in the mouse experimental autoimmune encephalomyelitis model of MS as evaluated by quantifying disability, histology, electron microscopy, and visual evoked potentials. Together, these findings support targeting M1R for remyelination and support further development of PIPE-307 for clinical studies.
Keywords: multiple sclerosis; muscarinic receptors; myelination; oligodendrocyte.
Conflict of interest statement
Competing interests statement:M.M.P., K.I.L., K.J.S., G.C.E., A.R.B., J.R.R., J.M.B., C.S.B., A.C.C., and D.S.L. are current employees of Contineum Therapeutics and hold financial shares of Contineum Therapeutics. Contineum Therapeutics owns patent rights to PIPE-307.
Figures


Comment in
-
Pipe dream: Remyelination therapy for multiple sclerosis.Proc Natl Acad Sci U S A. 2024 Aug 27;121(35):e2414324121. doi: 10.1073/pnas.2414324121. Epub 2024 Aug 19. Proc Natl Acad Sci U S A. 2024. PMID: 39159383 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
- R01MH125515/HHS | NIH | National Institute of Mental Health (NIMH)
- A130141/Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (AMRF)
- R01NS115746/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS115746/NS/NINDS NIH HHS/United States
- R01 MH125515/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

