Effects of in utero delta-9-tetrahydrocannabinol (THC) exposure on fetal and infant musculoskeletal development in a preclinical nonhuman primate model
- PMID: 39083456
- PMCID: PMC11290632
- DOI: 10.1371/journal.pone.0306868
Effects of in utero delta-9-tetrahydrocannabinol (THC) exposure on fetal and infant musculoskeletal development in a preclinical nonhuman primate model
Abstract
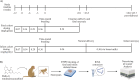
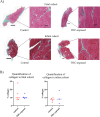
The endocannabinoid system (ECS) plays a major role in the maintenance of bodily homeostasis and adaptive response to external insults. It has been shown to regulate crucial physiological processes and behaviors, spanning nervous functions, anxiety, cognition, and pain sensation. Due to this broad activity, the ECS has been explored as a potential therapeutic target in the treatment of select diseases. However, until there is a more comprehensive understanding of how ECS activation by exogenous and endogenous ligands manifests across disparate tissues and cells, discretion should be exercised. Previous work has investigated how endogenous cannabinoid signaling impacts skeletal muscle development and differentiation. However, the effects of activation of the ECS by delta-9-tetrahydrocannabinol (THC, the most psychoactive component of cannabis) on skeletal muscle development, particularly in utero, remain unclear. To address this research gap, we used a highly translational non-human primate model to examine the potential impact of chronic prenatal THC exposure on fetal and infant musculoskeletal development. RNA was isolated from the skeletal muscle and analyzed for differential gene expression using a Nanostring nCounter neuroinflammatory panel comprised of 770 genes. Histomorphological evaluation of muscle morphology and composition was also performed. Our findings suggest that while prenatal THC exposure had narrow overall effects on fetal and infant muscle development, the greatest impacts were observed within pathways related to inflammation and cytokine signaling, which suggest the potential for tissue damage and atrophy. This pilot study establishes feasibility to evaluate neuroinflammation due to prenatal THC exposure and provides rationale for follow-on studies that explore the longer-term implications and functional consequences encountered by offspring as they continue to mature.
Copyright: © 2024 Moellmer et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Navarro G, Morales P, Rodriguez-Cueto C, Fernandez-Ruiz J, Jagerovic N, Franco R. Targeting Cannabinoid CB2 Receptors in the Central Nervous System. Medicinal Chemistry Approaches with Focus on Neurodegenerative Disorders. Front Neurosci. 2016;10:406. doi: 10.3389/fnins.2016.00406 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

